At-Home Overnight Artificial Pancreas Trial Reveals Health and Lifestyle Benefits for Adolescents
New research shows adolescents experienced improved blood-glucose control and quality of life when using an overnight artificial pancreas system for three weeks
Over the past several years, partially-automated artificial pancreas systems have transitioned from hospital testing to carefully monitored at-home trials, demonstrating their potential to improve blood-glucose control and quality of life for people living with type 1 diabetes (T1D). Now, JDRF-funded researchers at the University of Cambridge have taken testing one such system to the next step in the longest published outpatient trial of an overnight artificial pancreas system to be conducted in patients’ homes, and without strict monitoring by researchers. The study is part of JDRF’s broader program to advance the incremental development and delivery of increasingly sophisticated artificial pancreas systems. Such technology could help reduce the burden of living with T1D by automatically managing blood-glucose levels safely and effectively.
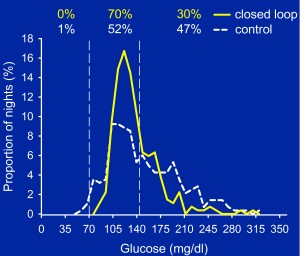
Published last month in Diabetes Care, the three-week study, led by Dr. Roman Hovorka, showed that use of an overnight artificial pancreas system led to improved blood-glucose control through the night—and residually through the next day—in 16 adolescent participants with T1D, ages 12-18. The system automates insulin delivery by increasing or decreasing insulin infusion based on monitoring of glucose levels every 12 minutes. The average nighttime blood-glucose levels of the participants remained in the target, safe range for 70 percent of the nights when the system was used, compared to 52 percent of the nights when the system was not used.
Percentage of Nights with Mean Glucose in Target Range (70-144 mg/dL)
Hovorka et al, Diabetes Care, May 2014
Furthermore, the number of nights with hypoglycemia decreased by nearly 50 percent. Hypoglycemia, or extreme low blood sugar, is a dangerous yet common occurrence for people with the disease. The researchers concluded that unsupervised home use of the overnight artificial pancreas system in adolescents with T1D is both safe and effective.
In addition to improved blood-glucose control, trial participants and their parents reported improvements in quality of life when using the system, which were outlined in a companion study by Katharine Barnard of the University of Southampton. Among the benefits noted were greater peace of mind without having to frequently monitor their blood-glucose, better sleep due to not needing to wake up through the night to check their blood-sugar levels, and more confidence in their diabetes control.
JDRF is committed to supporting longer-term studies that show sustained benefits in both blood-glucose control and quality of life, and continues to engage commercial partners to accelerate the development of such systems. Longer and more comprehensive studies could help pave the way toward bringing artificial pancreas systems to market for use overnight—when variables such as eating and exercise pose less of a challenge. This could be a valuable step toward the eventual commercialization of artificial pancreas systems that are safe and effective 24 hours a day.
For more information or to support JDRF’s artificial pancreas research program, please click here.