Stem Cell Replacement Therapy: Its Time has Begun
You know how JDRF talked about stem cell-derived precursors that can—once implanted in humans—mature into beta cells and produce insulin? That was ViaCyte’s PEC-Encap, VC-01™. And now, the company’s second technology, VC-02™, which is in a first-in-human clinical study, has preliminary results.
The trial yielded the first reported evidence of implanted stem cells secreting insulin in response to food/meal consumption, however, the insulin secreted by the implants did not have a sufficient clinical effect, i.e., the people in the trial still had to take insulin.
The trial results are also one of the first to report implanted cell survival and functionality one year post transplantation, which is significant for the durability of these treatments.
What’s next?
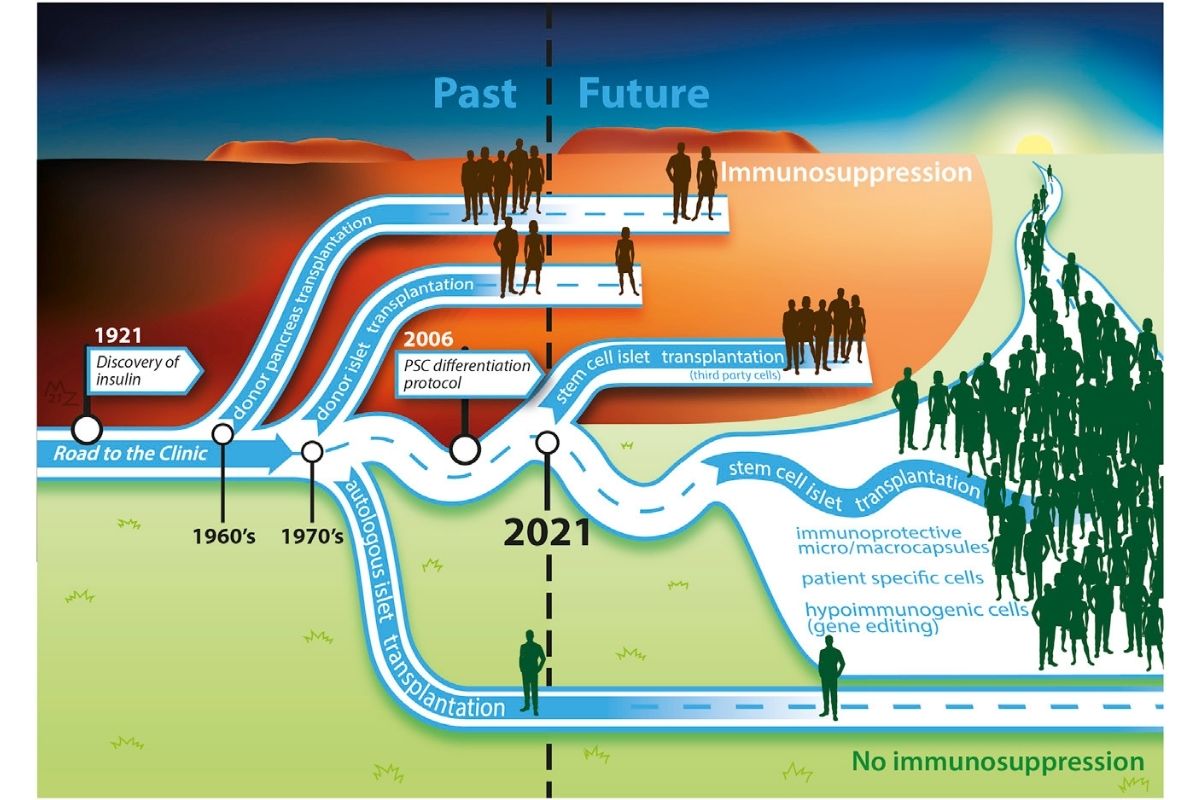
Many questions remain, as spelled out by Eelco de Koning, M.D., Ph.D., and Françoise Carlotti, Ph.D., in their accompanying commentary. Researchers need to determine the differentiation stage at which the cells are most optimal for transplantation, and the best transplantation site. It is also not clear whether the effectiveness and safety of the cells can be maintained over time, whether the dose of cells is sufficient, and whether it is possible to eliminate the need for immunosuppressive therapy.
But, they end with this: “An era of clinical application of innovative stem cell-based islet replacement therapy for the treatment of diabetes has begun.”
Yes, it has.
JDRF Leadership: JDRF has been a long-time and significant supporter of ViaCyte, supporting the company through research funding, as well as advocating for government funding for the California Institute of Regenerative Medicine, which also supported ViaCyte. Our funding 15 years ago (when ViaCyte was called Novocell) underwrote development of the proprietary line of precursor stem cells used in their treatment. JDRF also funded the preclinical and clinical studies of ViaCyte’s PEC-01™ therapies, which are designed to mature into islet tissue in humans, including glucose-responsive insulin-secreting beta cells. This includes the first ever clinical trial to test a stem cell-derived cell replacement therapy for T1D, in 2014.
Beta cell therapy is one of JDRF’s priority T1D cures pathways, and this clinical trial is one of a number of potential beta cell replacement cures therapies JDRF is supporting.
JDRF believes that stem cells are a promising way to create a cell replacement therapy. Read our stem cell therapy timeline for more info.
Photo Citation: de Koning and Carlotti, Stem cell-based islet replacement therapy in diabetes: A road trip that reached the clinic, Cell Stem Cell (2021), https://doi.org/10.1016/j.stem.2021.11.008