FDA Authorizes a Fourth Artificial Pancreas System
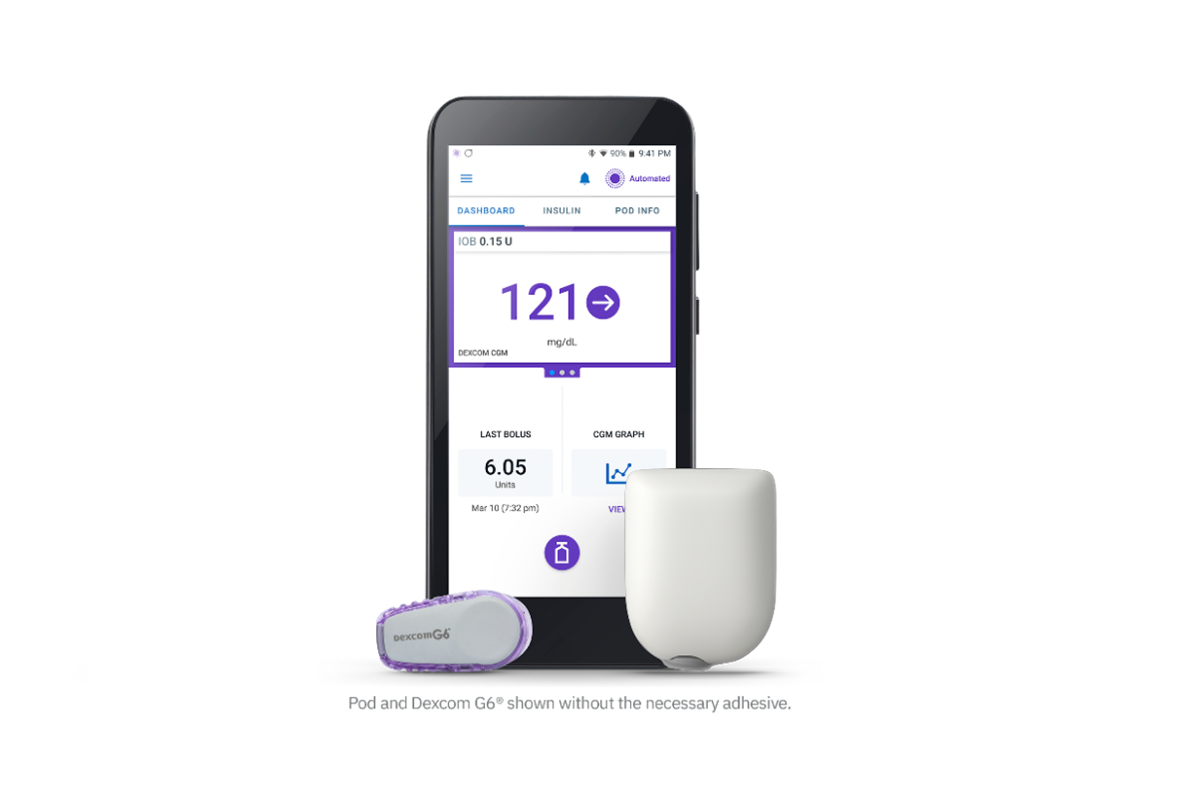
The Food and Drug Administration (FDA) today authorized the Insulet Omnipod 5, the world’s first tubeless, wearable system for individuals 6 and older. It includes an algorithm placed in a waterproof, closed loop insulin pump and communicates directly with a Dexcom G6 continuous glucose monitor (CGM).
There were three FDA approvals of artificial pancreas systems on the market: The Medtronic 670G, approved in 2016, the Tandem Control-IQ™, approved in 2019, and the Medtronic 770G, approved in 2020. This marks the first tubeless hybrid closed loop system to receive FDA authorization.
Data for Insulet’s submission of Omnipod 5 came from their Omnipod 5 Automated Insulin Delivery System pivotal trial. The trial included 128 adults and adolescents (14–70 years old) and 112 children (6–<14 years old) and demonstrated improvements in several key metrics, including a remarkably low rate of hypoglycemia:
- Adults and adolescents:
- Time in range (70–180 mg/dL) increased 9% from 65% to 74% compared to participants’ standard therapies (an additional 2.2 hours per day)
- Average HbA1c decreased from 7.16% to 6.78%
- Median time below 70 mg/dL decreased from 2.0% to 1.1%
- Children:
- Time in range (70–180 mg/dl) increased 15% from 53% to 68% compared to participants’ standard therapies (an additional 3.7 hours per day)
- Average HbA1c decreased from 7.67% to 6.99%
- Median time below 70 mg/dL remained at 1.5%
This data demonstrates that this system will help people with T1D achieve improved glucose control—a key goal of JDRF’s Improving Lives program.
JDRF Impact
JDRF’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, JDRF developed a roadmap for artificial pancreas development with increasingly advanced versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- JDRF started the Artificial Pancreas Project over 15 years ago to ensure people with T1D have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The JDRF Artificial Pancreas Project and the JDRF Artificial Pancreas Consortium have dramatically accelerated progress by bringing academic researchers, government agencies, industry, and the Helmsley Charitable Trust together to pursue artificial pancreas technology.
JDRF has funded over $135 million to date in artificial pancreas research.
- JDRF supported work in this area through investigators in the JDRF Artificial Pancreas Consortium, Francis (Frank) Doyle, Ph.D., Eyal Dassau, Ph.D., and Howard Zisser, M.D., and their colleagues at the Sansum Institute (California). They created the first algorithm, which was eventually licensed by Insulet Corporation that led to the development of Omnipod 5.
- Industry experts have said JDRF’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system in 2016, the first approved artificial pancreas system.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.
Per Insulet, “Omnipod 5 is expected to be broadly available shortly after the limited market release.”