Verapamil Slows Type 1 Diabetes Progression in Newly Diagnosed Children and Teens
To one day cure type 1 diabetes (T1D), we must halt the destruction of beta cells that produce insulin. A new JDRF-funded study suggests a potential path to keeping beta cells healthier for longer—meaning their body will still make insulin for more time, known as the “honeymoon” phase—for newly diagnosed youth.
The clinical trial looked at whether the effects of a hybrid closed loop system (also known as an artificial pancreas system or automated insulin delivery system) and/or verapamil preserved beta cell function one year after diagnosis in children and teens with T1D.
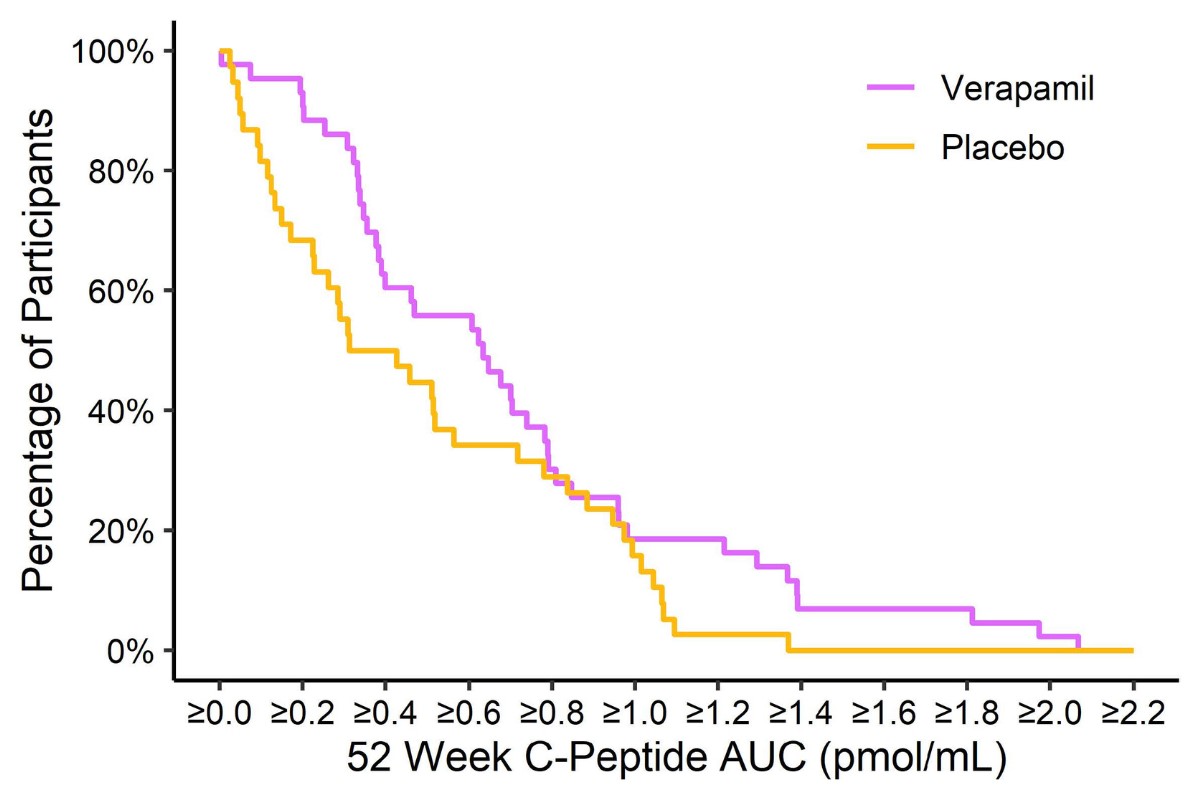
The study found that newly diagnosed individuals on verapamil were making more insulin one year after diagnosis than those on placebo, with the average C-peptide, which is used to measure insulin, being 30% higher for the verapamil group compared to placebo. HbA1c was 6.6% in the verapamil group versus 6.9% in the placebo group, at one year. (There was no change, however, in the hybrid closed loop system arm.)
“Safe, effective therapies are urgently needed to delay disease progression in people recently diagnosed with type 1 diabetes, an area of high priority for JDRF,” said Sanjoy Dutta, Ph.D., JDRF chief scientific officer. “The CLVer study is the second trial showing that verapamil, an inexpensive and widely used blood pressure medication, can preserve beta cells in the new onset period, making us one step closer to our goal of having disease-modifying therapies widely available for people with type 1 diabetes.”
But you don’t have to rely on what the blog said; from the clinical study authors: “oral verapamil was well-tolerated and slowed the rate of beta cell decline in youth with newly-diagnosed type 1 diabetes….In view of the favorable safety profile…once-a-day oral administration, and low cost, initiation of verapamil therapy should be considered for newly-diagnosed type 1 diabetes.”
What Does It Mean for the T1D Community?
Today, verapamil is not an approved therapy for newly diagnosed people with T1D, and it will not be in the very near future. There are additional studies that may need to be conducted to validate the results and learn so that all the benefits of the drug are known, as well as all of the potential side effects. JDRF has a strategic road map to answer all these questions. These include:
- What are the long-term effects of the drug?
- Does the preserved beta cell function last?
- Can this drug help people in stage 2 T1D?
There’s a lot more we must learn!
What Comes Next?
JDRF will gather longer-term evidence of verapamil’s effectiveness, while in the near term sharing these data with the clinical community and other health care leaders to facilitate future access:
- JDRF has a grant for a follow-up study for three years to see if C-peptide benefits persist
- JDRF is funding several clinical trials to validate the results of this study and see if verapamil is effective when used in conjunction with other disease-modifying therapies, such as Tzield™ (teplizumab-mzwv)