FDA Approves Medtronic 780G Artificial Pancreas System
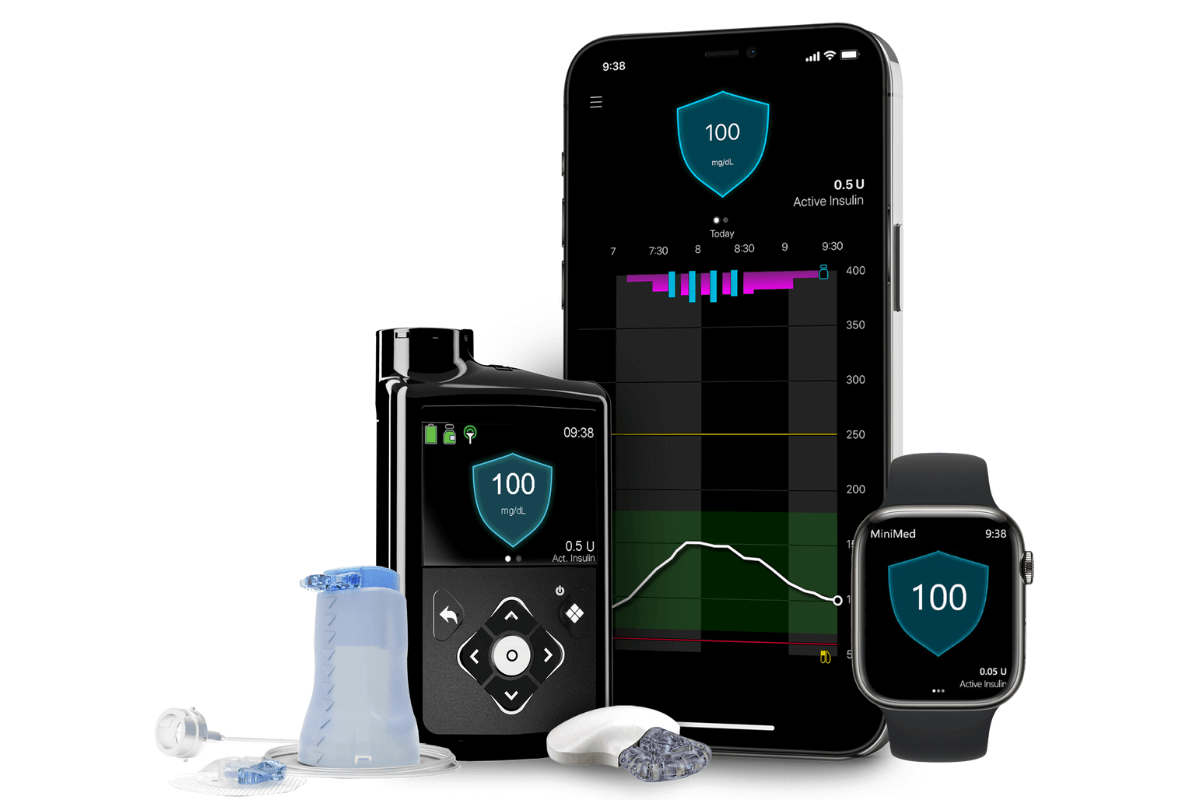
The Food and Drug Administration (FDA) recently approved the Medtronic MiniMed™ 780G artificial pancreas system for use in individuals 7 years and over. It provides automatic adjustments and corrections to blood-sugar levels every 5-minutes, and also correction doses, as part of its meal detection technology.
This is an evolution of the 670G, the world’s first artificial pancreas system, which was approved in 2016, and an updated version, 770G, which was authorized in the United States in 2020. (The 780G was approved in Europe in 2020.)
In the pivotal clinical trial:
- Time-in-range (blood sugar between 70-180 mg/dL) was 75 percent, helping those with T1D maintain more consistent and healthier glucose levels
- The overall time-below-range was 1.8 percent, and overnight it was 1.5 percent
- Overnight time-in-range was 82 percent
The 780G is approved for use with the Medtronic Guardian 4 continuous glucose monitor (CGM). Unlike its predecessor, the Guardian 3, the Guardian 4 does not require fingerstick calibrations.
The 780G is also approved for use with the Medtronic Extended Infusion Set (EIS), which can be used for seven consecutive days (most infusion sets can be used for 3 days).
Per Medtronic, individuals will be able to pre-order the device on May 15 with an expected delivery in June.
JDRF Impact
JDRF’s strategy focuses on improving lives and cures through research and advocacy to accelerate therapies through the pipeline. Through these efforts, JDRF developed a roadmap for artificial pancreas development with projections for next generation versions of the artificial pancreas. Manufacturers embraced the roadmap to guide their own research and development programs.
- JDRF started the Artificial Pancreas Project almost 20 years ago to ensure that people with T1D would have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation.
- The JDRF Artificial Pancreas Project and the JDRF Artificial Pancreas Consortium have dramatically accelerated progress by bringing together academic researchers, government agencies, industry partners, and the Helmsley Charitable Trust to pursue artificial pancreas technology.
JDRF has funded over $140 million to date in artificial pancreas research.
- Five algorithm teams of the JDRF Artificial Pancreas Consortium and Tidepool developed algorithms that made the Medtronic MiniMed 670G, 770G, and 780G, Tandem Control-IQ™, CamAPS® FX, Insulet Omnipod 5, and Tidepool Loop
- Industry experts have said JDRF’s involvement cut five years off the approval process for the Medtronic 670G artificial pancreas system, the first artificial pancreas system, which was approved in 2016.
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management until cures are found.