FDA Clears a New Artificial Pancreas System
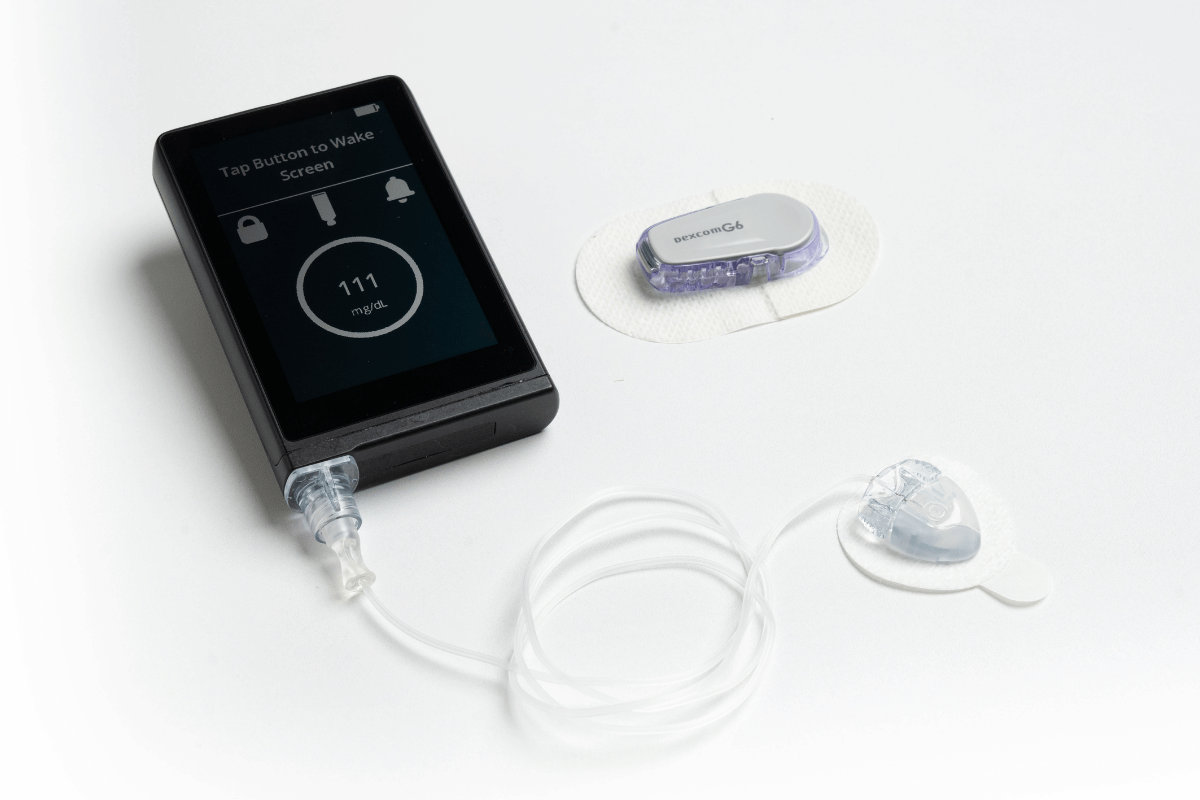
The U.S. Food and Drug Administration (FDA) cleared the iLet® Insulin-Only Bionic Pancreas System, which is designed to autonomously determine and deliver insulin doses to control blood-sugar levels, for people 6 years of age and older with type 1 diabetes (T1D). It includes an algorithm and an integrated infusion pump, which communicates directly with a compatible FDA-cleared integrated continuous glucose monitor (iCGM), enabling it to be an artificial pancreas, or automated insulin dosing (AID), system.
What’s new about this system? Ease-of-use. The iLet system is designed to have users enter only their weight for the iLet to initialize therapy. Immediately thereafter, the iLet begins controlling blood-sugar levels automatically, without requiring the user to count carbohydrates, set insulin delivery rates, or deliver additional insulin for meals or corrections. (Users do have to say whether the amount of carbs in a meal is small, medium, or large, but the algorithm learns over time in response to their individual insulin needs.)
The submission was based on a multi-center randomized insulin-only iLet Bionic Pancreas pivotal trial, which tested the insulin-only configuration in 440 adults and children 6 years and older with T1D. The trial met all key endpoints, demonstrating improved outcomes over standard of care for people living with T1D:
- Average HbA1c fell from 7.9% to 7.3% at 13 weeks
- An average of 2.6 hours more time-in-range (70-180 mg/dL) per day, improving from 51% to 65% at 13 weeks
- No increased risk of hypoglycemia
There are now multiple artificial pancreas systems on the market: The Medtronic 670G (2016), Tandem Control-IQ™ (2019), Medtronic 770G (2020), Insulet Omnipod 5 (2022), Medtronic 780G (2023), and, now, the iLet® Insulin-Only Bionic Pancreas System. (Tidepool Loop, an app that contains an algorithm that automates insulin dosing, has also been approved, but it has not yet announced its insulin pump manufacturer.)
JDRF Impact
JDRF started the Artificial Pancreas Project over 15 years ago to ensure people with T1D have better, more innovative ways to manage their T1D until there are cures. Our goal was to ensure life-changing options for people with T1D and a competitive ecosystem that drove continuous innovation. To date, JDRF has funded more than $140 million in artificial pancreas research.
Through these grants, JDRF supported the development of the algorithm and preclinical and early clinical research—in partnership with the Helmsley Charitable Trust—through grants to:
- Firas El-Khatib, Ph.D., who received a JDRF postdoctoral fellowship from 2006-2007 and is now co-founder and VP, Research & Innovation, at Beta Bionics
- Ed Damiano, Ph.D., co-founder and executive chair of Beta Bionics, from 2009-2011
- Steven J. Russell, M.D., Ph.D., who received a grant from 2013-2016 and is the principal investigator on all of the iLet clinical trials
This is a win for the T1D community and provides people with T1D another option to improve daily blood-sugar management, until cures are found.