Scientific Sessions 2018: More in store for people with T1D

By Gary Scheiner, MS, CDE
 As usual, this year’s American Diabetes Association (ADA) Scientific Sessions didn’t place much emphasis on a healthy lifestyle. There was food everywhere—and trust me, we’re not talking fruits and veggies. And given the oppressive Orlando heat and humidity, the only running we did was to get a seat near the A/C in the various lecture halls. But unlike most ADA conferences, this year’s meeting featured many new and exciting approaches to type 1 diabetes (T1D) care.
As usual, this year’s American Diabetes Association (ADA) Scientific Sessions didn’t place much emphasis on a healthy lifestyle. There was food everywhere—and trust me, we’re not talking fruits and veggies. And given the oppressive Orlando heat and humidity, the only running we did was to get a seat near the A/C in the various lecture halls. But unlike most ADA conferences, this year’s meeting featured many new and exciting approaches to type 1 diabetes (T1D) care.
Here’s a quick summary:
What… A Pill?
Finally, the pharmaceutical industry is paying some attention to the needs of the T1D community. Sanofi, in partnership with Lexicon Pharmaceuticals, presented data from preliminary studies on use of sotagliflozin (brand name Zynquista—a big-point word if you’re playing scrabble)—an SGLT-1 and SGLT-2 inhibitor in adults with T1D.
SGLT-1 inhibitors blunt the absorption of glucose through the upper-GI tract, thus delaying the glucose rise after meals; SGLT-2 inhibitors block the re-absorption of glucose by the kidneys, thus leading to excretion of glucose in the urine. The two together have a synergistic effect, producing a significant reduction in A1C, post-meal glucose peaks, weight, and bolus insulin requirements. Users saw their “time in-range” (as measured by CGM) increase from 56 to 68 percent. Sanofi submitted sotagliflozin to the FDA for a T1D indication back in May and hopes to have approval sometime in 2019.
CGM Dominance
For the first time ever at a major diabetes conference, there were more companies promoting continuous glucose monitoring (CGM) systems than traditional glucose meters—and with good reason. Continuous glucose monitoring is no longer a “luxury” for those who can afford it; it has become the standard of care for just about everyone who uses insulin.
Maryland-based Senseonics received a great deal of attention with their three-month glucose sensor that is implanted just below the skin on the back of the arm. Its accuracy is even better than that of Dexcom (and without weekly change-outs and warm-ups), and the sensor implantation process is a lot less invasive than everyone imagined.
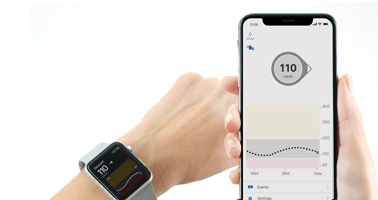 Abbott was busy selling the merits of its Libre system as a replacement for finger sticks. But without high/low alerts and an inability to be calibrated, Libre is running into resistance in the T1D marketplace.
Abbott was busy selling the merits of its Libre system as a replacement for finger sticks. But without high/low alerts and an inability to be calibrated, Libre is running into resistance in the T1D marketplace.
Dexcom proudly showed off its new calibration-free (but calibration-capable) G6 system with one-button sensor insertion. Even Medtronic got back into the act with its new Guardian Connect freestanding CGM system, emphasizing its pattern-recognition software. Regardless of the brand, if you have T1D and are not yet using a CGM, you’re really missing out.
Closing the Loop
Traditional insulin pump therapy was completely overshadowed by hybrid closed-loop (HCL) technology. The only real innovation with the pumps themselves involved Insulet’s recently-approved Dash programmer, a cell-phone-like device that will replace the bulky, old-school Personal Diabetes Manager (PDM) for controlling the OmniPod using Bluetooth communication.
But there was greater excitement over data on Insulet’s Horizon HCL system, which links the Dexcom G5 sensor with algorithm-enabled pods. Safety and performance data on the system’s personalized, model-predictive control algorithm showed an increase in time spent in-range (from 64 to 74 percent) and a reduction in time spent in a hypoglycemic range—both overall (from 5 to 2 percent) and overnight (from 6 to less than 1 percent).
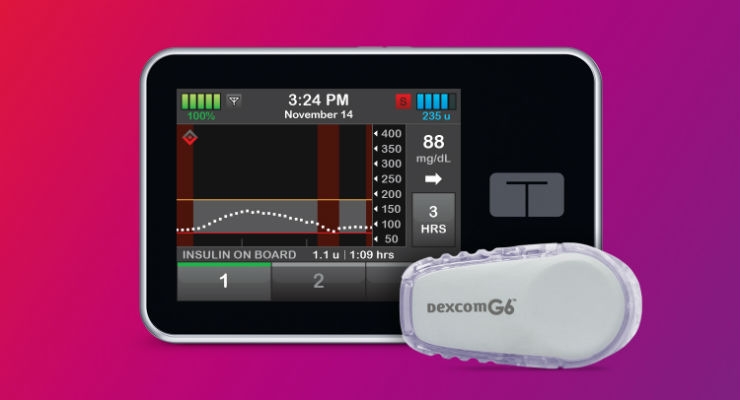 Tandem presented data on its recently-approved Dexcom G6 connectivity and predictive low glucose suspend (Basal IQ) feature, which suspends basal insulin delivery any time glucose levels are projected to dip below 80 mg/dl within the next 30 minutes. Basal delivery resumes as soon as glucose levels rise at all. The feature, which runs in the background, does not disturb the user and turns basal insulin off/on automatically. While reducing the incidence of hypoglycemia by 31 percent, it was not associated with any increase in average glucose.
Tandem presented data on its recently-approved Dexcom G6 connectivity and predictive low glucose suspend (Basal IQ) feature, which suspends basal insulin delivery any time glucose levels are projected to dip below 80 mg/dl within the next 30 minutes. Basal delivery resumes as soon as glucose levels rise at all. The feature, which runs in the background, does not disturb the user and turns basal insulin off/on automatically. While reducing the incidence of hypoglycemia by 31 percent, it was not associated with any increase in average glucose.
Medtronic continued to report positive outcome data (increases in time-in-range and reductions in hypoglycemia) on its 670G HCL, which combines Medtronic’s algorithm-enabled pump with its Guardian sensor. Outcomes aside, there were multiple sessions focusing on strategies for overcoming many of the hassle factors associated with the system, causing some to look for alternatives.
Two such options are offspring of the “We Are Not Waiting” movement: The Loop app and Open APS. Both are do-it-yourself systems developed as freeware to be used by anyone with the technical expertise to build/install them correctly. Because these systems were not subject to the scrutiny and limits imposed by the FDA, they offer much greater customization and more aggressive glucose management algorithms than the commercially-available systems.
Sci-Fi for MDI
For those who choose to stay on injections rather than use a pump, there is a new device that helps to bridge the technology gap. InPen, from Companion Medical, is a Bluetooth-enabled insulin pen that links directly with a unique smartphone app. The pen uses disposable Humalog or Novolog insulin cartridges and delivers in half-unit increments—but the app is what really makes it special.
All doses delivered by the pen are transmitted to the app for automatic logging purposes. And that’s not all! The app aids the user in calculating mealtime doses in a manner similar to an insulin pump: carbs and blood sugars are entered, and the app determines the dose less insulin-on-board from previous doses. And that’s still not all—several meters and CGM devices transmit glucose readings directly into the app. And (you get the idea)—the app prompts the user to take and log their long-acting insulin. Logbook and statistical reports can also be generated in PDF format.
Faster, Better Insulin
With the launch of Novo Nordisk’s Fiasp insulin earlier this year, we now have something faster-acting than the traditional Humalog/Novolog/Apidra we’ve been using for decades. Fiasp contains an extra ingredient (niacinamide) which makes it absorb faster through the fat layer below the skin, resulting in a slightly earlier onset of action and peak action time. But don’t get too excited; the new formulation only works an average of eight minutes earlier, so it is still necessary to pre-dose at most meals.
Lilly isn’t far behind. By combining Humalog with a vasodilator, they can accelerate the absorption and action of the insulin as well. A third company, Adocia, is working on combining rapid-acting insulin with an enzyme (hyalaronidase) that effectively “loosens” the fat layer to produce faster absorption. Insulin combined with hyalaronidase starts working an average of eight minutes earlier, peaks 10 minutes earlier, and stops working 22 minutes earlier than ordinary Humalog. It also shows greater action one hour after injection and a shorter duration of action compared to Fiasp.
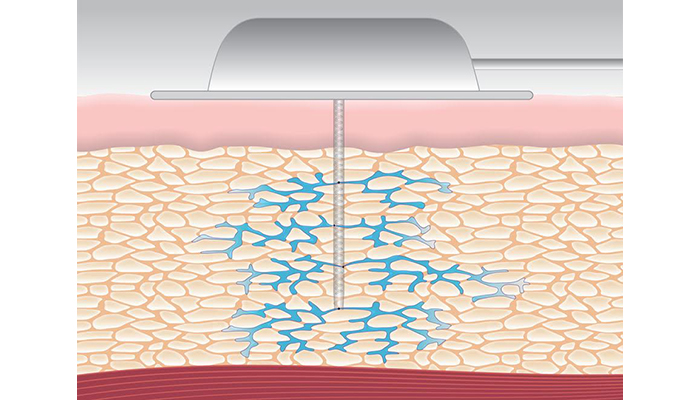 And finally, a startup called Capillary Biomedical is busy developing a novel insulin pump infusion set that features a kink-free cannula with multiple openings through which insulin can flow. The design produces less inflammation at the insertion site, fewer occlusions, and wider/more even insulin distribution in the subcutaneous fat layer. Their goal is to produce an infusion set that lasts much longer than current sets with minimal variation in insulin absorption.
And finally, a startup called Capillary Biomedical is busy developing a novel insulin pump infusion set that features a kink-free cannula with multiple openings through which insulin can flow. The design produces less inflammation at the insertion site, fewer occlusions, and wider/more even insulin distribution in the subcutaneous fat layer. Their goal is to produce an infusion set that lasts much longer than current sets with minimal variation in insulin absorption.
Making Use of Technology
All of these innovations sound wonderful, but none are worthwhile unless they produce the kind of results we all want: better glucose control and less interference in our daily lives. Applying technology is the key, and that requires a motivated user and the guidance of a skilled professional.
Certified Diabetes Educators (CDEs) are health experts with specialized training in diabetes. If you don’t work with a CDE, check with your physician or local hospital, or visit ncbde.org/find-a-cde. Our practice is based near Philadelphia but offers remote consulting services for people with T1D and their families worldwide. Feel free to contact us with any questions at 877-735-3648 (610-642-6055 outside North America) or email me directly at gary@integrateddiabetes.com.
A Final “Frigid” Note…
Apparently, praying to the Ice Gods at the Orlando Ice Bar (a giant frozen room with a full-service bar)—even with the combined efforts of myself and JDRF Chief Mission Officer Aaron Kowalski—does not guarantee a speedy cure for diabetes (see image below).

________________________________________________________________________________________________________________________________

About the Author
Gary Scheiner is owner and clinical director of Integrated Diabetes Services, an AADE-accredited, Wynnewood, PA-based practice specializing in intensive insulin therapy. Gary is author of seven books, including the award-winning “Think Like a Pancreas,” and was named AADE Diabetes Educator of the Year in 2014.