JDRF FUNDING OF CELL THERAPIES

Minnesota & Dakotas Clinical Trial List
JDRF Clinical Trials Connection Tool
Type 1 diabetes (T1D) is a progressive disease that we now know has several stages. JDRF is working on having therapies for all stages of T1D. We currently use screening in stages 1 and 2 to detect antibodies before stage 3, which is clinically diagnosed T1D. Future disease modifying therapies hope to halt, prevent, and reverse stages 1 through 3. Cell therapy research addresses stage 3 T1D in the newly diagnosed and in those who have had T1D for many years and no longer have beta cell function. JDRF studies research differently at each stage, yet all research is connected in T1D. Early-stage research also has an impact on the later stages. Early research investigates how immunity recognizes beta cells as targets. JDRF also works to bring advances in other fields of research on autoimmunity, to T1D targeting of cells and tissue in T1D.
The goal of new cell therapies in stage 3 is to use live cells to treat or reverse T1D. JDRF is accelerating cell therapies toward commercialization by supporting preclinical and clinical testing, and by helping with regulatory pathways along with access to these therapies. The goal is to deliver externally derived beta cells or islets to restore insulin independence and blood glucose control.
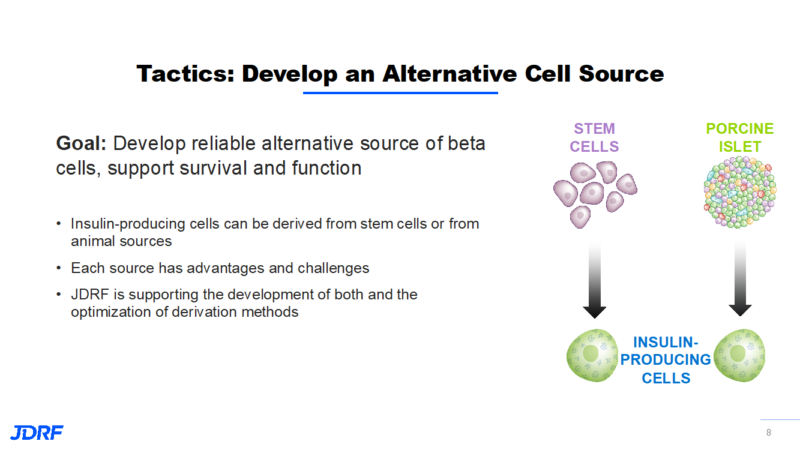
Currently, islet cell transplantation requires immunosuppression which may lead to more susceptibility to infections. With this requirement in mind, those currently receiving transplants must be experiencing hypoglycemia unawareness. JDRF’s goal is to develop new therapies with renewable cell sources that will reduce the need for insulin, immunosuppression, and encapsulation. The cure strategy in cell therapies is to develop reliable sources of beta cells and to support their survival and function while shielding beta cells from immune destruction. JDRF is looking at insulin-producing cells that are derived from stem cells or from animal (porcine/pig) sources. Each source has advantages and challenges. JDRF is supporting the development of both types of cells. When looking at sources, we need to provide enough cells for everyone who needs them. An animal source is more abundant, but individuals may have more of a risk for a strong immune response. Researchers will need to study at which stage of cell maturation these cells would be used. For example: would they be from neonatal, juvenile, or adult pigs? Human stem cells would not have as strong of an immune response. There is still work to be done on how they go through the differentiation process for all to become fully functioning beta cells. This will include finding the optimal stage for these cells to be implanted. JDRF is supporting both types of new islet cells.
The next step would be to decide the optimal location in the body to place new cells. Multiple sites are being explored such as skin, muscle, and the abdominal cavity. The formation of blood vessel networks that can provide nourishment to implanted cells will be key. Currently, the liver is the site for islet cell transfusions. This may not always be ideal, as these cells can cause liver inflammation and this may alert the immune system. The liver is also an organ that filters out toxins from our diet and our environment. These toxins may be toxic to the newly transplanted cells. This environment may be responsible for a significant number of newly transplanted cells becoming non-viable. We need to have a better site that is safer for the cells and that can help us with retrieval if needed. Blood vessel formation is key for cells to receive nutrients and oxygen, and to be able to respond to glucose and then send insulin back into the bloodstream.
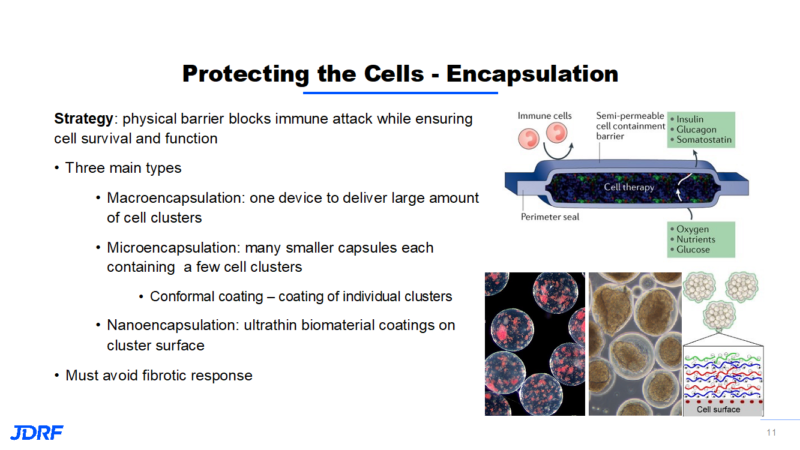 Encapsulation provides a physical barrier that blocks the immune attack while ensuring cell survival and function. There are three main types of encapsulation (see above). The drawback of the large macroencapsulation device is that the concept relies on diffusion, which is a very slow process. The field sees promise in moving to microencapsulation to improve transport of nutrients. Microencapsulation increases the surface area and decreases the volume size. Researchers are working on ways to make this type of transplant of multiple capsules more retrievable. This has led to conformal coating, which is coating individual clusters. With nanoencapsulation, the idea is to deposit an ultrathin coating on the cluster surface. This also improves the transport of nutrients.
Encapsulation provides a physical barrier that blocks the immune attack while ensuring cell survival and function. There are three main types of encapsulation (see above). The drawback of the large macroencapsulation device is that the concept relies on diffusion, which is a very slow process. The field sees promise in moving to microencapsulation to improve transport of nutrients. Microencapsulation increases the surface area and decreases the volume size. Researchers are working on ways to make this type of transplant of multiple capsules more retrievable. This has led to conformal coating, which is coating individual clusters. With nanoencapsulation, the idea is to deposit an ultrathin coating on the cluster surface. This also improves the transport of nutrients.
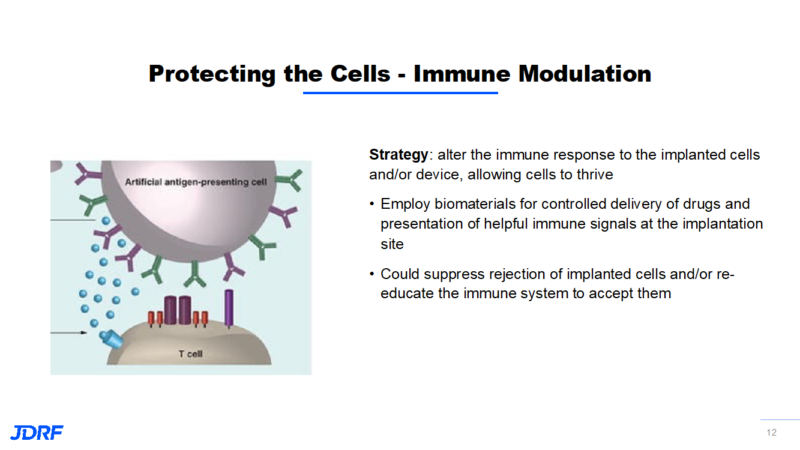
Some of the newer technologies involve protecting new insulin-producing cells from being recognized by the immune system, or improving their environment for their survival. A new strategy is to use gene editing tools to enhance the survival and function of implanted cells. Genes control cell function by controlling the production of substances like proteins. By targeting genes and the proteins they control, we can intervene in the immune recognition, autoimmunity, and tolerance process. Gene editing is used to knock off the surface markers on a cell so the immune system does not recognize this cell as a beta cell. It becomes camouflaged. It is now possible to target cells and proteins to make the environment more robust and enhance their function to help them survive. Besides gene editing, other types of immune modulation to help the implanted cells survive are being studied.
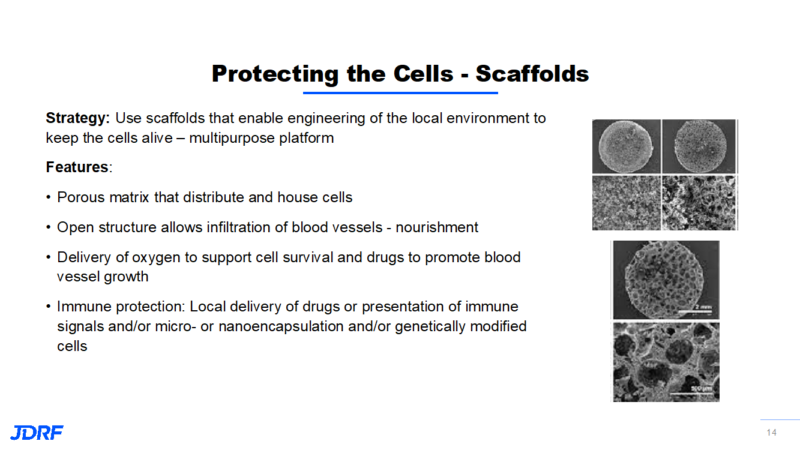
Another way JDRF is funding research that protects new beta cells is with the concept of scaffolding. The idea is to use scaffolds that are engineered to provide a protective local environment to keep cells alive. It will have multipurpose features that can provide for new cells. Using a scaffold falls in between immune enhancement and immune regulation and will help cells survive. This matrix is like a sponge with open pores. This can also provide protection for the cells from each other so they do not clump together and compete for the same nutrients. This makes it easier for cells to get nutrients and oxygen in order to survive. This open structure allows for the development of blood vessels for nutrients and for the interaction with the blood glucose levels and the secretion of insulin. The scaffolding also allows delivery of oxygen during the time of cell placement along with a way to deliver drugs that may help cell survival.
JDRF is supporting research across early discovery and through translational research. JDRF supports early clinical development and advises on regulatory strategies for the most promising and advanced cell therapies for T1D. JDRF has a very strong advocacy program that also works on regulatory issues that help to get new therapies to patients. JDRF also works alongside academia and industry. Academia often works with discovery and development of novel approaches and strategies. By catalyzing partnerships between academic investigators with promising technologies and industry partners capable of developing commercial products, we can work together in funding, in infrastructure, and with regulatory strategies.
JDRF has helped build consortiums in various T1D research fields in order to create a scientific environment where ideas, along with real progress, can be shared. The beta cell replacement consortium is one of these groups. JDRF believes a cure will require expertise from multiple sources. This consortium has more than 50 members. The consortium can work together to:
*Identify and test novel biomaterials
*Design better macro devices, capsules and scaffolds
*Standardize data to be consistent and transferrable across investigators
*Translate results from small to large animals and humans
*Optimize local immunosuppression approaches
Here are some of the highlights in the portfolio of cell therapies we have funded or are currently funding:
*Viacyte has three devices for microencapsulation that are currently being studied. They are able to detect C-peptide, a marker of insulin production, in the cells they have developed.
*Vertex has purchased SEMMA with two parallel programs. One is a cell implant with immunosuppression (no device) that hopes to enter clinical trials in 2021. The second is an encapsulated cell implant with no immunosuppression, which is in preclinical development.
*Sernova is a Canadian company that is pursuing the scaffold-like approach for new beta cells. Their approach works by priming the skin prior to cell implantation to enhance cell survival. There have been promising preliminary clinical trial results in January 2021. This has included no adverse events observed, device safe and well tolerated, device integrated well, and demonstrated ingrowth of blood vessel networks. This is needed for the flow of nutrients for the cells, and for cells to respond to blood glucose values in order for them to secrete the appropriate amount of insulin back into the bloodstream. Indications of clinical benefit such as reduction in daily insulin requirement and C-peptide in the blood have been observed.
*Novo Nordisk along with Procyon Technologies is a collaboration on the development of an encapsulation cell therapy that has come out of this consortium. The approach being used is to provide oxygen to a microencapsulation device in order to enhance device capacity and cell survival. This pairs Procyon Technologies’ oxygen-enabled encapsulation devices and Novo Nordisk’s stem cell-derived insulin-secreting cells. It will focus on further optimization of the device and cells to accelerate preclinical development and entry into the clinical phase. JDRF played a critical role in supporting early development of technology and catalyzing this partnership.
*Researchers Andres Garcia of Georgia Tech and Haval Shirwan of the University of Missouri are collaborating to develop a biomaterial-based immune modulation approach. This approach uses biomaterials for local delivery of immune signals to suppress immune system rejection. They have demonstrated prolonged survival of implanted cells and acceptance and long-term function when combined with a short-term immunosuppression used only in the first two weeks after transplant.
*Researchers Stephen Kissler and Peng Yi at the Joslin Diabetes Center are using gene editing to prevent autoimmune responses to implanted cells. They have identified multiple genes that make mouse beta cells vulnerable to autoimmune attack. By blocking the function of these genes, we can protect the beta cells from attack. They are testing blocking combinations of genes to enhance the effect and to validate this approach that can be used with human cells. By employing gene editing to modify the beta cells prior to implantation, this can prevent autoimmunity. Their work can also lead to new drug-based therapies.
The main focus of the cell therapy projects is to find alternative sources of beta cells along with new approaches for delivery and immune protection of these cells. JDRF is catalyzing the establishment of new partnerships to accelerate development and commercialization to accelerate entry into clinical translation. Multiple companies are showing promising early results in the clinic and gaining critical experience.
There continue to be many clinical trials in our chapter area with a variety of types of trials such as drug and device, along with others. Here is the chapter chart showing the active clinical trials. As always, if you have any questions, please contact me, Debbie Evans. Cell: (612) 810-1933 or email at: debbieaevans1@gmail.com.