JDRF’s Improving Lives Program – Complications and the Psychosocial Aspects of T1D
Minnesota & Dakotas Clinical Trial List
JDRF Clinical Trials Connection Tool
The subject of the psychosocial impact of Type 1 Diabetes (T1D) is a heart wrenching topic to discuss. Those of us with family members living with T1D know how exhausting it is to constantly think of T1D 24/7. We all know someone, who at some point, has struggled with the mental health aspect of a chronic disease. JDRF’s mission is to improve the lives of people living with T1D by accelerating the development of advanced drugs, devices, behavioral interventions, and combinations of these to improve short-term and long-term health outcomes and quality of life. JDRF is making psychosocial well-being a priority. JDRF’s goal in the psychosocial area is to have effective diabetes-focused behavioral interventions available to people with T1D to treat mental health issues, and to increase access to psychological care and support by increasing the number of trained psychology professionals in the field of T1D. Twenty-five percent of people with diabetes experience depression at some point in their lives. Adults with T1D who have depression also have a higher HbA1c than individuals without depression. Psychological interventions are available, but have limitations such as long-term effectiveness, accessibility, and affordability. Another limitation is that there are not many interventions available. Youth with T1D show a higher suicide rate. In adolescent girls, 7% with T1D meet diagnostic criteria for an eating disorder – a rate twice as common as in girls without diabetes. In youth, 14% with diabetes reported mild depression and 8.6% reported moderate-to-severe depression. JDRF’s strategy in psychosocial is to:
• Standardize measures of psychological outcomes
• Validate and move interventions toward broad implementation for
those with T1D
• Increase the number of trained psychology professionals
A good way to look at improving the psychosocial aspect is to look at patient related outcomes (PRO) measures that can provide unique information on the impact of a medical condition and its treatment from the patient perspective. There needs to be more healthcare standardization and quality improvement that provides more personalized medicine. JDRF supports studies to develop and refine outcomes and implement them in clinical practice through clinical trials. Several JDRF goals that look at PRO are:
• PRO development for hypoglycemia
• PRO development for stigma
• Enhance standard set for adults
• Develop standard set for children
• Gain a global consensus and buy-in partnership and collaboration with new and existing partners
JDRF is trying to include more PROs in clinical trials to add how treatments can help patient well-being. JDRF will continue to work with stakeholders to help this happen.
JDRF is looking at a precision medicine and holistic approaches to T1D for both physical and mental health complications. By integrating all these components via complex artificial intelligence analysis, we can create profiles and personalize treatments. At the Elizabeth Weiser-Caswell Diabetes Center at the University of Michigan, researchers are working to identify patterns and describe the relationship between metabolic profiles, psychosocial states and cognitive function, and cognitive function. This will help to define the metabolic profiles associated with a variety of organ complications such as diabetic retinopathy (DR), diabetic nephropathy (DN), and neuropathy. Several approaches need to be considered due to complications having various time horizons and that fact that researchers can’t use a one-size-fits-all approach.
Family support and intervention is an important consideration, and JDRF is studying the well-being of the family. It has been found that higher levels of parental distress are associated with worse youth outcomes, including higher rates of youth depressive symptoms, worse quality of life, and more diabetes-specific family conflict. Parents report high levels of diabetes-specific emotional distress, with prevalence rates as high as 34% during this first year with parents feeling isolated, too. JDRF is supporting the efficacy testing of a telehealth intervention designed to improve family well-being. Chapter support groups help and JDRF wants to develop more of this kind of help.
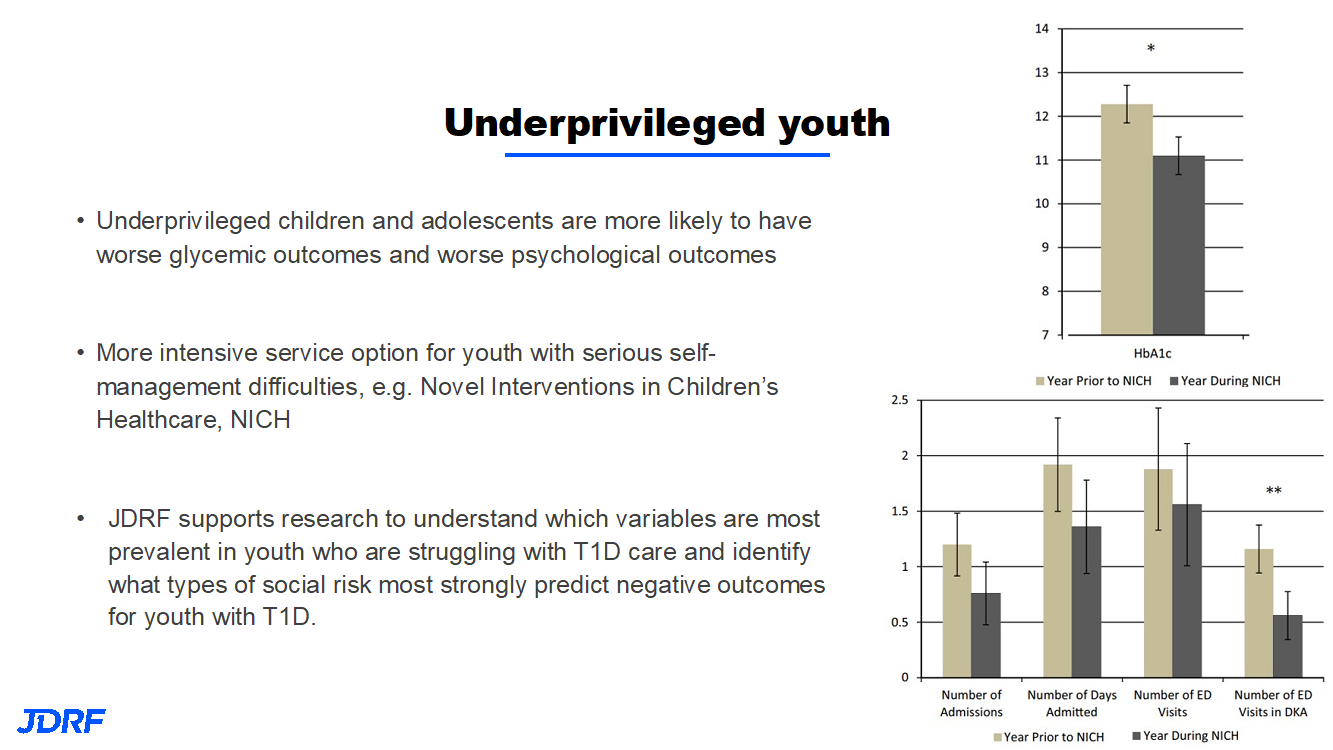
JDRF is working to provide better outcomes, both for psychosocial and better glycemic outcomes for underprivileged youth. More intensive service options are needed for youth with serious T1D self-management difficulties. One such example is the Novel Intervention in Children’s Healthcare, which helps families with stretched incomes improve their child’s health by improving community health, healthcare, and reducing healthcare costs. Poverty, food, and housing insecurity can impact self-management, as these are multiple challenges. JDRF is partnering with the Helmsley Charitable trust in this work.
Telepsychology and access not only work better to have better outcomes in the T1D population, but can also help with underprovided youth. Telehealth can enable people who would otherwise have limited access to care to manage their T1D and mental health. It can help address stigma for patients seeking psychological services for the first time and can improve retention. JDRF is supporting the development and deployment of a virtual care platform to improve mental health for adults with T1D in rural and remote areas. This year has shown that this approach can be beneficial.
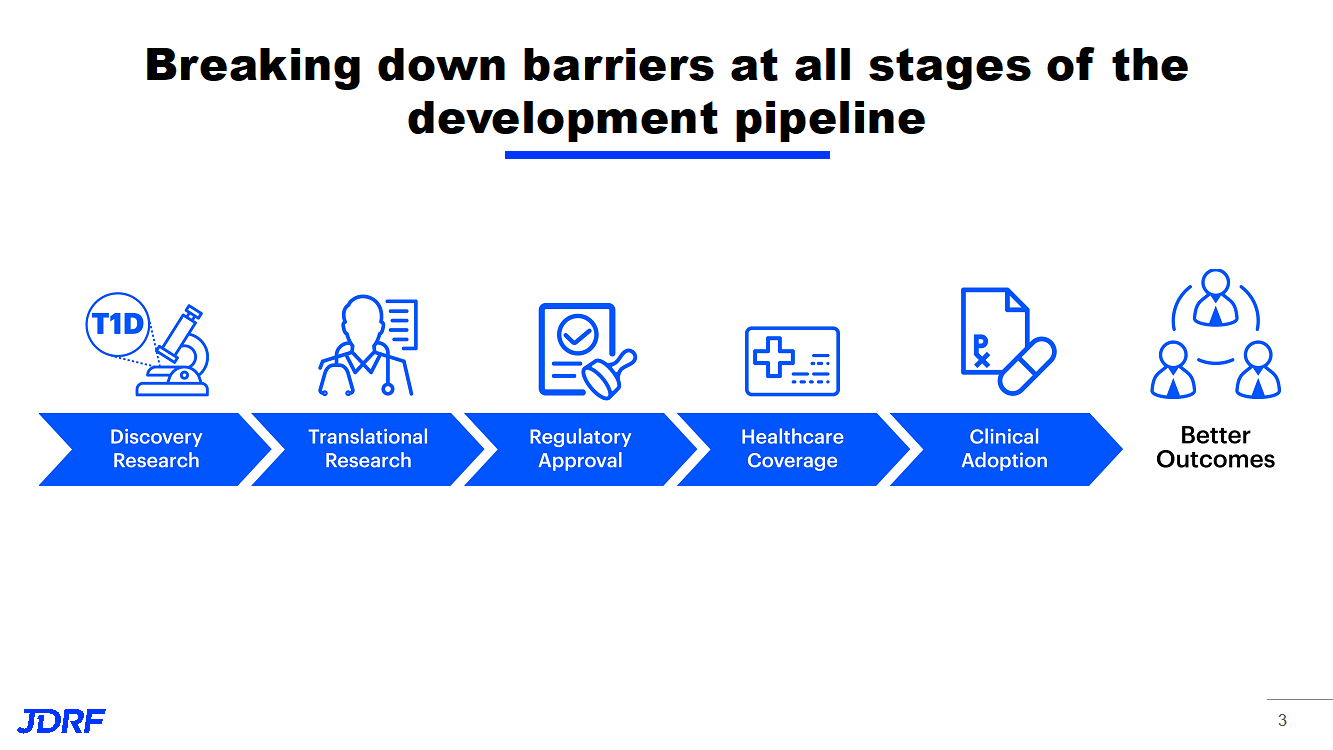
JDRF’s vision is a world where people with T1D live healthy and burden-free lives. There are unmet needs to address in order to accomplish these goals. Clinically, blood glucose (BG) control is still a challenge; broader metabolic control unaddressed by current therapies and complications remains a reality for many with T1D. There are also unmet mental health and quality of life needs to study and improve. People with T1D are disproportionately affected by mental health issues, which can affect T1D outcomes. The burden of this disease, such as the inconvenience of therapies, careful monitoring of diet, and insulin administration, imposes a toll on all people with T1D. JDRF’s improving lives program is developing therapies to improve health and reduce the burden of living with T1D.
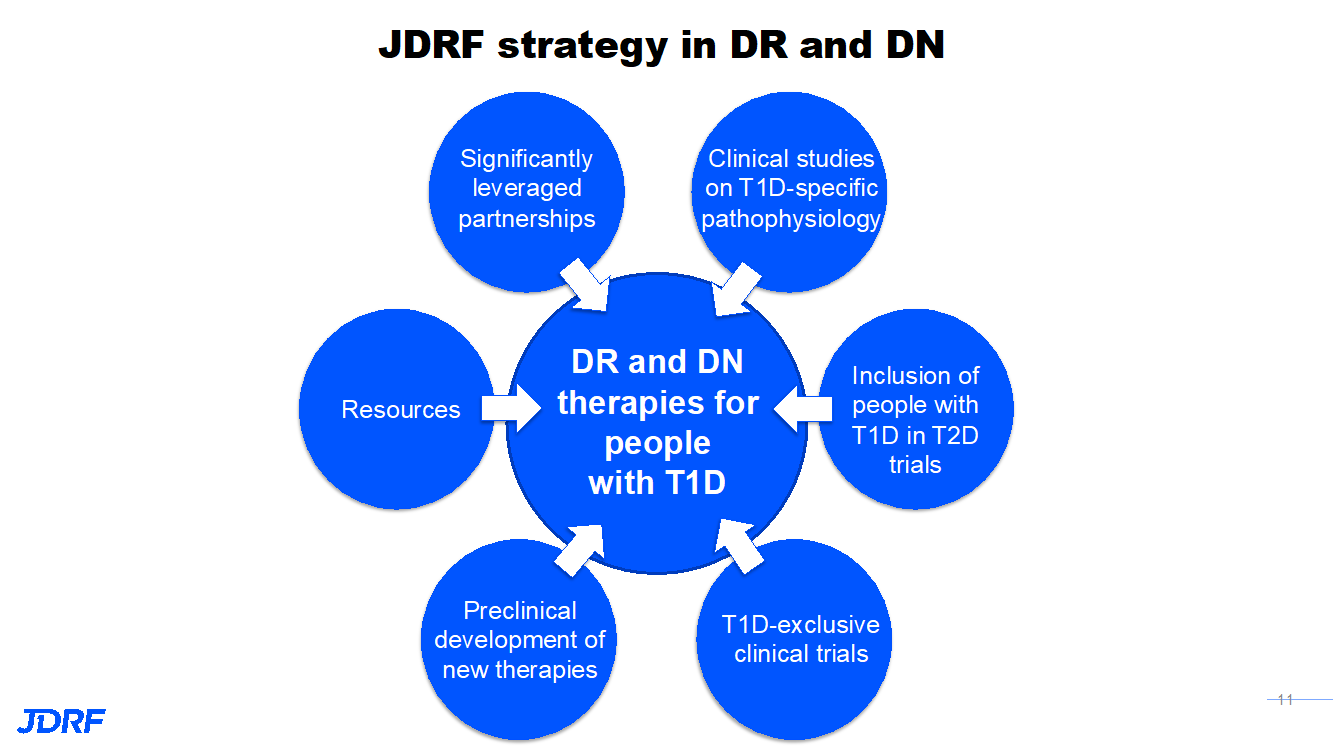
The artificial pancreas (AP) program continues to work on better AP components and systems. This includes faster insulins, other drugs to combine with insulin, drugs for complications, and therapies for psychosocial burden. Currently, we have effective treatments such as Lucentis and Eylea for diabetic eye disease, but there are no targeted therapies for kidney disease. There need to be more study-validated psychosocial interventions for those with T1D. For eye disease, diabetic retinopathy (DR), a long-term complication, can cause vision loss. In one study, after 25 years, 97% had some changes to the eye, and in some, changes that can precede vision loss. This did not mean that these were changes that participants in the study were aware of, or that affected their vision. Approved therapies represent a major advance, but treatments have limitations and work is still needed. Kidney disease, diabetic nephropathy (DN), is a long-term complication of T1D that affects more than half of the T1D population. We do now have ace inhibitors that are helping slow or prevent DN, but we need better treatments. The good news is that T1D complications have gone down over time with better ways to keep BG in control.
In order to move forward on successful treatments, JDRF is also able to leverage partnerships that help bring more resources to the table and facilitate getting treatments to patients. The natural progression of eye and kidney disease occurs over a long period of time. This makes it harder to develop effective treatments and necessitates partnerships. Clinical Trials have to be long to detect success in treatments for eye and kidney disease that take so many years to develop. This is why we need these partnerships of academic centers, companies, and government. In this way, JDRF can reach its lofty goals.
JDRF, in partnership with the Mary Tyler Moore and S. Robert Levine, MD Foundation, are working to prevent the progression of vision loss and restore vision already lost in DR. Work is being done to develop a new staging and severity scale for eye disease to enable efficient product development. This will ultimately help develop interventions that can reverse vision loss.
Clinical trials for DR with VEGF inhibitors have been done and further studies are in process. Thanks to JDRF funded research, Lucentis and Eylea (VEGF-inhibitors) are approved for DR. They are administered after eye disease has developed. The next step is to see if earlier, less frequent use of these VEGF inhibitors can prevent onset of macular edema and proliferative DR. This may improve vision outcomes with lower side effects by using these drugs less frequent. It is always better to prevent a complication than to treat an existing complication.
Currently, the standard of care for DR is burdensome, costly, and is less than 100% effective. The drug fenofibrate is a generic, convenient, safe, and cheap treatment that has the potential to transform clinical care. JDRF is supporting a definitive clinical trial testing fenofibrate in DR for T1D and T2D. This is in collaboration with DRCR, NIH, ROCHE and the Helmsley Charitable Trust.
For diabetic nephropathy (DN), in order to develop new therapies, a better understanding of T1D DN and its pathophysiology is required. JDRF supports clinical studies to understand how DN occurs in people with T1D. These studies will also look at kidney biopsies to see what is occurring so researchers can develop the best drug for prevention. The TRIDENT and KPMP consortia, using cutting-edge science to analyze kidney biopsies, are working on the discovery of new therapeutic tools for DK. Other drugs to be studied for DK in T1Ds include GLP1 (e.g. Ozempic), SGLT inhibitors (e.g. Farxiga/Dapagliflozin), and MRA (e.g. Finereone). They have been studied in those with T2D.
JDRF is funding earlier stages of the product development pipeline, supporting preclinical work on novel drugs and drug delivery methods that will improve eye and kidney disease outcomes. Examples of this include novel anti-fibrotics for eye and kidney disease, novel anti-inflammatory drugs for kidney disease, and T-reg (T regulatory cells) for eye disease.
The Improving Lives program is developing drugs, devices, and behavioral health interventions to improve outcomes and quality of life for people with T1D. Improving the lives of people with T1D remains a top priority for JDRF. JDRF is prioritizing T1D complications that affect the kidney disease and eye disease. JDRF is also able to leverage partnerships globally to address these complications. JDRF’s goal in the psychosocial area is to have effective diabetes-focused behavioral interventions available to people and families living with T1D.
In our chapter there is currently a variety of trials that have treatments being studied that are either drug, device or another intervention that would be considered to be research that improves the lives of those with T1D and addresses complications. Here is the chapter chart showing the active clinical trials. As always, if you have any questions please contact me, Debbie Evans. Cell: (612) 810-1933 or via email at: debbieaevans1@gmail.com.