Prevention screening is a major priority for JDRF

Screening to prevent T1D is one of JDRF’s major priorities. This will include advocating for expansion of screening not only in the United States but also in the global community. Countries such as Germany, Finland, Sweden and Australia have been active in the screening of those with and without a connection to T1D. Our main study in the US for screening is TrialNet. Screening and learning that someone is at risk for developing T1D may enable a child or adult to have other study options that may delay or prevent development of a new case of T1D. We are at a new frontier. A pivotal study showed those who were in stage 2 of T1D, when given teplizumab, were able to delay T1D onset by two years. You may have heard about this several years ago in JDRF literature. As these participants continue to be followed, we now know that delay of T1D is at three years.
Today our screening is mostly familial and this captures only 10-15% who need to be screened. When a person is newly diagnosed approximately 60% are in DKA at that time. Those who have been part of a screening study and are being followed for development of T1D have a much lower chance of DKA around 3%. JDRF sees a future where the general population both children and adults can be screened as part of the healthcare system where it is available to all. Along with global expansion of screening for T1D, we will then pick up many more at risk of getting T1D and will be able to provide more disease modifying therapies and treatments for a cure. There are currently 7 treatments that are being looked at to help delay, prevent and cure T1D, one is teplizumab. We know that the development of T1D occurs in stages, with the first stage often happening many years before stage 3 and clinical diagnosis of T1D. With this in mind, this is why I as a parent of a child with T1D do consider research on prevention also to be research that is for a cure. We are getting closer to being able to delay or prevent T1D before it becomes the insulin-dependent stage 3. See this link to sign up for TrialNet.
Read more
The benefits of screening go further than lowering the chance of DKA at diagnosis. We currently know that reducing and avoiding DKA gives a better A1c outcome, reduces the need for a hospital stay, and improves long-term glucose control and improved cognitive function. We are now able to see many disease-modifying therapies launching to slow, halt or reverse the course of T1D. In order to test these therapies in a clinical trial, there needs to be more screening to find those that are in early development of antibodies and on the path to T1D in order to have an impact. There are many other treatments being tested, but our current screening programs miss most people at risk such as those in the general population. Many who develop T1D have no known family connection. We need to increase the number of people getting screened and to help advocate for a change in public policy so more robust screening of the general population is covered by healthcare. The advancement of the JDRF mission also involves screening. Our mission which states “Improving lives today and tomorrow by accelerating life-changing breakthroughs to cure, prevent and treat T1D and its complications is our mission”. Increasing the availability of screening will help accelerate disease-modifying therapies through the pipeline by; increasing the number of people eligible for clinical trials, identifying people eligible for disease modifying therapies when available and improve near and long-term health benefits for those screened.
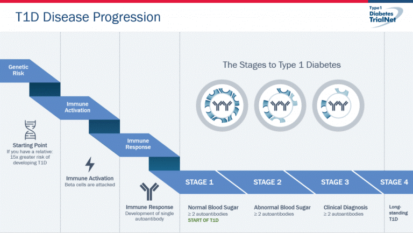
We now know that T1D starts early in life. We know there is a large genetic component that alters immunity. It is known that a series of genes can contribute to your risk of beta cell dysfunction and autoimmunity. At some point in your life you have a trigger that activates your immune system to attack yourself and in this case your pancreas. Once you have developed two or more autoantibodies you are in stage 1 and this will eventually lead to T1D in your lifetime if no intervention. Once it is detected that your glucose levels are elevated by metabolic tests, but not yet needing insulin, you are in stage 2. Once you have lost a major percentage of your beta cells due to the immune attack and need insulin to lower your glucose level you have entered stage 3 and now have the clinical diagnoses of T1D.
As mentioned, there are a number of drugs in development and different treatments will target different stages, which is another reason why screening is an important part of this process. Teplizumab was shown to be successful in stage 2 for instance.
Trialnet (US), ASK (Colorado), ENDIA (Australia), BABYDIAB (Germany) are leading the charge in longitudinal studies of at-risk individuals and clinical trials for prevention. A longitudinal study is a prospective observational study that follows the same subjects repeatedly over a period of time. With screening we can chart the progression of T1D, support T1D families, identify and help mitigate barriers to participation in clinical studies and help advance the first potential preventative therapy for T1D. First-degree family members have a 1-20 chance of developing T1D. In the general population it is 1 – 300. Being part of a screening study can also help to reduce the burden of risk and helps to reduce the psychosocial part of knowing that you are at risk.
In the familial screening study in the US, TrialNet, has screened 15,000 per year with 750 (5%) being identified as having markers leading them towards becoming T1D. This is about 1 in 20. The ages that are being screened are from 2-45 depending on if you have a first or second-degree relative with T1D. The TrialNet staff does the work on this study and NIH-SDP, JDRF, and Helmsley Charitable Trust (HCT) help to provide funding. ASK, a screening trial in Colorado, screens both those at risk and those in the general population, this study also is tracking celiac disease. So far ASK has screened 60,000 and has picked up 560 (1%) who have markers for development of T1D. The percentage of those picked up as at-risk for T1D development is lower than TrialNet as ASK is also testing those in the general population. ASK is funded by JDRF, HCT and Janssen.
Here is what we know about what enhances the risk of T1D. We now can accurately predict the risk of getting T1D due to the presence of autoantibodies. We now have additional tools added to risk assessment criteria that can help more precisely determine the time frame for disease development. Some of these criteria involve age, analyzing up to 41 gene sites, environmental and geographic factors, and other circulating blood markers. For instance, we know that those with 2 or more autoantibodies are 100% sure to get T1D at some point in their life if no intervention. At five years it is 40%, ten years is 70%, fifteen years 80%. We cannot say exactly when someone may become insulin dependent. With additional test, we are now able to offer clinical trials with treatments that are showing promise in halting or delaying the progress of the disease to clinically diagnosed T1D. This is one of the major reasons to consider enrolling family members that qualify and have not yet developed T1D but have a relative with T1D. For Minnesota, Wisconsin, North Dakota and South Dakota, there is a TrialNet site at the University of Minnesota. This trial can be initiated online with no travel. Please click here: Trialnet to find out how to enroll in this study. You can also contact Shannon Beasley at 612-626-5609 or at beasl103@umn.edu.
We are now seeing a path to success in preventing this disease and we can get there through the help of participants in clinical trials. TrialNet is for those who have not yet developed T1D. For those who may have had a very recent diagnosis (within three weeks), there is also an important study at the U of Minnesota called CLVer. This trial is hoping to impact the disease when first diagnosed and is why you must call and talk to the study staff as soon as you learn of the diagnosis. It is a difficult time when a family has a new diagnosis of T1D, but it is important to find out about these studies for those newly diagnosed. These studies hope to improve near and long term outcomes of this disease. For a child with a new T1D diagnosis within the last three weeks you can also contact Shannon Beasley at 612-626-5609 or reach her at beasl103@umn.edu.
Please feel free to reach out to me if you have any general questions about clinical trials or JDRF research. I will be glad to either answer your questions or find the answer from a large network of JDRF research staff. Reach out to me, Debbie Evans, at 612-810-1933 or email me at debbieaevans1@gmail.com.