T1Dectect / Clinical Trials and Health Policy RIV Talk
This article is covering the research information volunteer (RIV) talk that occurred recently and presented by Anastasia Albanese-O’Neal PhD., APRN, CDCES, Director of Community Screening and Clinical Trial Education, and Aaron Turner-Pfiffer, Director, Health Policy.
JDRF has universal global screening as one of its top five research priorities. The reason for this comes out of the research that has shown that type one diabetes (T1D) occurs in stages. These stages were accepted as medical standards of care in diabetes in 2015. JDRF led this effort to stage T1D, and specifically stage the presymptomatic stages. It is known there is a genetic risk along with some environmental trigger or interaction with the environment. What is seen is an immune response resulting in the production of proteins in the blood, which are autoantibodies, setting the stage for T1D. It is now possible to identify individuals at risk for what we call clinical T1D or stage three many years in advance of clinical disease.
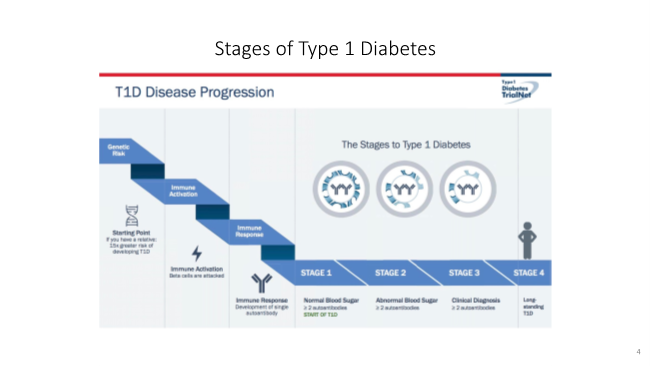
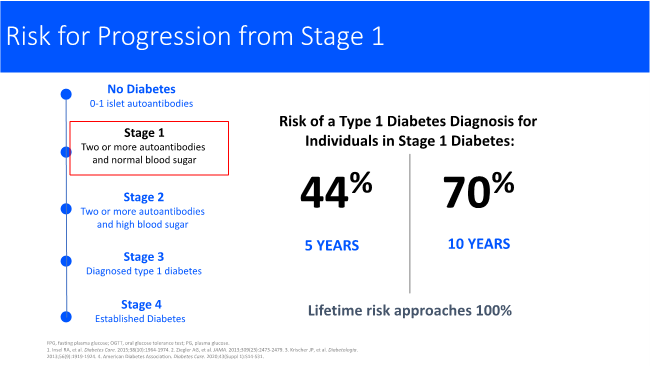
When blood is tested, we are looking for these autoantibodies. If zero to one autoantibody is detected the person has no diabetes and no increased risk. With stage 1, there are two or more persistent autoantibodies to the insulin producing cells in the pancreas. It is now known that this person over their lifetime will progress to stage 3 clinical T1D. In stage 1, people will have a normal blood sugar. Stage 2 is where there are two or more autoantibodies, and high blood sugar, usually after meals. Stage 3 is the classic diagnosis of T1D usually with symptoms. At some point after established T1D, there is no remaining beta cell mass. This is why stage 1 is very important. It is known from long standing studies that the risk of progression at the five -year mark shows 44% have gone on to develop T1D, at the 10-year mark 70% will be diagnosed with stage 3 T1D and require insulin. The lifetime risk approaches 100%. In stage 2, is where we see dysglycemia or hyperglycemia usually following meals. When stage 2 occurs the risk of T1D diagnosis accelerates. We see the time compressed to 60% of people who will be diagnosed in two years and 75% in five years. Again, the lifetime risk approaches 100%. When we can find people in stage 1 and 2, this is when there is the opportunity to intervene to slow or halt the disease. We cannot know if people are forming these autoantibodies in the presymptomatic stages unless they are screened. This is why a screening program is so important.
There is T1Detect which is an education and awareness program around T1D risk, screening and monitoring. Monitoring is important because when we screen, we can identify autoantibodies proteins in the blood that tell us that the insulin producing cells are under attack. The progression can take 2, 5, 10 sometimes 20 years. This is why we want to monitor people so T1D can be prevented and prevent loss of beta cells at diagnosis. JDRF is engaging people in this research area. The JDRF screening strategy has three pillars. There is the research piece, the T1Dectect outreach and education piece, and the health policy piece which will be covered later in this article. The overall goal is to build evidence for global universal screening or non-familial risk screening and enhance the accuracy and really understand how often people should be screened. JDRF’s long-term goal is to expand screening to all individuals, regardless of age, ethnicity, or their geography. The emphasis needs to be on monitoring and continuing to follow people and engage them in this clinical research and their own clinical care. JDRF wants to support and use all of this evidence to support policy evidence to marshall for policy change.

T1Detect as our guiding principles will reduce the incidence of diabetic ketoacidosis (DKA) at diagnosis. It is important to identify individuals for prevention or disease modifying therapy research studies. The first disease modifying therapy, teplizumab(Tzield), for T1D was approved recently. DKA rates are high at diagnosis in the United States. DKA prevention at diagnosis alone is sufficient to drive the importance of the T1Detect program. In the United States, DKA rates at diagnosis are between 40 and 60%. For children and adolescents, they are higher among racial and ethnic minorities. The rates in the United States are much higher than countries in Europe and Australia. We do lack data, especially for adults. We do see when following children and adolescents who are diagnosed in DKA, they do have higher overall lifetime glucose levels.
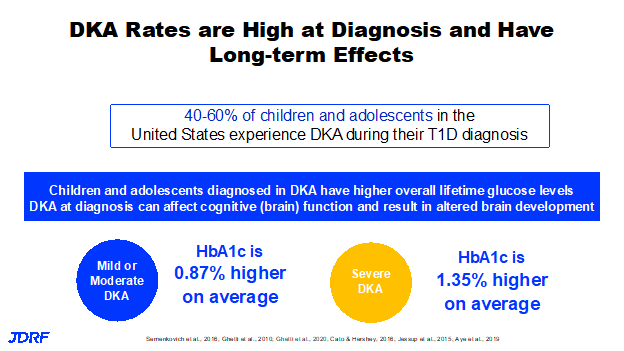
Also, in this group when diagnosed in mild or moderate DKA, the affect over their lifetime is just under a percent higher on average. If they are in severe DKA, meaning they have a very low blood PH at diagnosis, the affect is significantly higher, 1.35% higher on average over their lifetime. It is known that higher glucose levels are tied to complications over the long term. DKA at diagnosis can affect cognitive or brain function, and result in altered brain development. That comes out of the TrialNet studies. It is a call to action to reduce the number in DKA at diagnosis. JDRF also believes that people need the information to make an informed decision about screening so they can understand the pathway. A step-by-step roadmap is critical and that starts with screening. JDRF wants people to be able to identify the best pathway for screening along with information that can help you make the decision for yourself or your child to be screened. If you are eligible for research such as TrialNet, that is likely the best pathway. This will give you access to experts in the field, with no cost involved. This participation can be done from anywhere and if you do need to come into a study site you may receive minor compensation for your time and travel. If you are not eligible for research that does screening, there are other pathways available. You can find this information also at T1Detect. JDRF wants people to get screened and then have their results reviewed with their healthcare professional, whether it is an endocrinologist or a primary care provider.
This map shows that we have a lot of work to do in terms of making sure that when someone takes those results to a healthcare professional, particularly in primary care, that the healthcare professional is armed with the knowledge to help guide someone though the next steps in terms of monitoring. There is much to be done to get information out to healthcare professionals about the stages of T1D. For instance, pre-symptomatic staging of T1D is not part of nursing curriculum. If people are seen by nurse practitioners, primary care MD or DO’s, we need to make sure they have the information to support their patients, develop an individual monitoring plan. The plan for a two year old who screens is going to be different from that of a 20 year old and different from a 70 year old. JDRF would like to see ongoing evaluation and engagements so we can get people to clinical studies so we can prevent DKA at diagnosis. Again, education and awareness for people in the T1D community and for healthcare professionals is a pillar of JDRF. We want people to know the research pathway for screening, but also to know they can use clinical labs such as Glassdoor LabCorp or another resulting agency along with the option of a home pathway. You can also use a Enable Biosciences kit. There is work being done to help with monitoring support as to what needs to be done after screening. Peer support is something JDRF can provide because there is a lot of people in our organization who have walked this road and can provide meaningful peer support. With looking at monitoring guidelines, the goal is to improve outcomes and accelerate disease modifying therapies.
The screening recommendations is a living guideline as the more we learn through research the more we can improve these guidelines. For instance, the American Diabetes Association (ADA) in 2021 made a big change to the standards of medical care and diabetes. Prior to 2021, screening was only recommended in the research setting. The screening recommendations have expanded to first degree relatives of individuals with T1D. This is new as there has not been a lot of screening done in the clinical space. This is a key opportunity and a long term goal of JDRF to expand screening and find those with autoantibodies before they have clinical T1D. There is certainly a call to action for global universal screening. In this fiscal year, JDRF is looking at a more intense focus on that recommendation in the clinical guidelines for the familial screening and specifically for first degree relatives. It is estimated that there are about 1.4 million Americans living with T1D, we know from data provided by NIH in the research setting that about 250,000 have been screened. This leaves millions of first-degree relatives in the United States alone that have never been screened. We need to move forward, work together and help get information out about this screening. First degree relatives have a 15 times higher risk for a diagnosis of T1D.
JDRF is working to help educate healthcare professionals, JDRF staff, all our volunteers and the T1D community more broadly to talk about screening and its importance. JDRF is also expanding work in clinical pilots to reach more diverse populations. Work is being done to enhance information online and in print, so that people can easily understand why they should get screened, how to get screened and what are the next steps to take. JDRF is working with partners, key opinion leaders, academics and other organizations to get consensus on monitoring guidelines, which currently do not exist. There are guidelines around screening, but not around guidelines after the screening is done. JDRF is working to close that gap. JDRF will be attending many of the conferences for diabetes educators, nurses and the American Diabetes Association (ADA) conference in June to share information meant to help everyone become more familiar with presymptomatic T1D. The dialogue will be directed at the attendees coming to these conferences and we will be working with our strategic partnerships in these efforts. JDRF is also exploring tutorials that can also provide work continuing education points (CME) for healthcare professionals. This would help to get information to everyone where they live.
There is also the opportunity to educate people through LinkedIn and Twitter where JDRF has seen a lot of interest in the sharing of JDRF information on this topic. JDRF is working with extraordinary key opinion leaders such as Michael Haller, M.D., from the University of Florida, Chantal Mathieu, M.D., PhD, who is the incoming leader at EASD and Kimber Simmons, M.D., MS, who is at the Barbara Davis Center. Kimber Simmons is doing some excellent work around providing monitoring support for follow up when autoantibodies are found. Internally JDRF is working with staff, volunteer constituents and the at large group of people affected by T1D. This is to offer events where you participate in a social event that has a learning component also. These will occur quarterly. JDRF wants to make sure that every person at JDRF is also up to speed on what is going on with global universal screening.
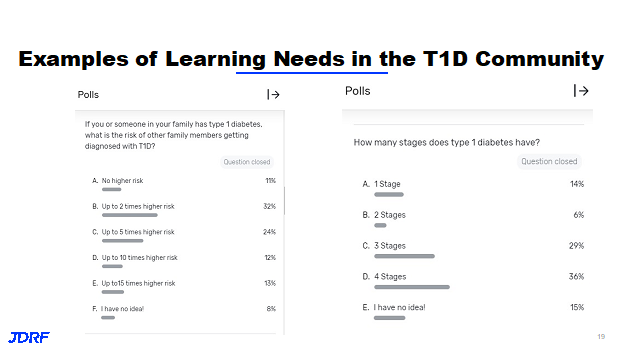
An example of this is a virtual Type One Nation (TON) summit this past October that was well attended where people were engaged and participating. In 2023, the goal is to have an educational component at ten of these TON events around the country where there will be discussions about screening. This is being presented in an interactive way. This way there will be some Q and A where there can be presentations of both familiar material and also things people need more information about. It will have a fun interactive format, similar to a game show including prizes. Look for notices for these new events and please attend. In the polling that JDRF has done at recent virtual events, many who attended were interested in screening. In the interactive quiz only a small minority of attendees knew the correct answers on screening and that if you have a family member with T1D you have a 15 times higher risk of developing T1D. There was a lot of confusion around the staging of T1D. Fifteen percent of the participants said they had no idea how many stages there were and many thought there was only 1 not 3. JDRF is working to help make sure people understand the staging and understand the importance of screening.
There are new pilot sites opening to help reach more communities. The first is at a large university affiliated hospital at Rutgers University. The children and families seen at this clinic speak a number of languages, including English, but also includes Spanish, Portuguese, Creole and Urdu. Since there is no TrialNet site here, this provides a real opportunity to bring screening to families who might not have been invited in the past and make sure that we are partnering with the clinic. This is run by the clinic and they are providing the why and how of screening and then also the follow up monitoring. This clinic, reaches 100% racial and ethnic minorities and has screened nearly 150 individuals. They are providing patient materials in English and Spanish. They have hired a bilingual patient navigator to make sure information is more accessible and to help families not only with screening, but other aspects of diabetes care. In this setting there is often a sole practitioner, pediatric endocrinologist. There is not the luxury of having diabetes educators and psychologists. We are hoping not only that we can educate about screening, but also provide additional support. At the other end of the spectrum, we have the Adams County Health Clinic, which is in a small town called Council, Idaho. It is a town of about 900 people. It has a rural Federally qualified health center and JDRF is helping by subsidizing screening test kit so screening can take place. This again is a group who might not be invited to screening. JDRF is helping this clinic to build internal and external capacity for screening and follow up monitoring. This clinic is also partnering with an organization called “Ask the Experts” at the Barbara Davis Center in Colorado. The center can provide remote follow up monitoring to this clinic, which is nurse led, and helps to give this clinic additional support. JDRF has identified additional sites for fiscal year 23 and will be activating them this year. There is exploration into staffing models that can work to support families and help them with making decisions in a clinical setting. This is also with keeping in mind the recommended guidelines after screening are still being worked out. JDRF is helping to create evidence to help with these new guidelines and then partner with investigators in academia and those also in the clinical care setting. There is testing of patient facing materials to make sure then make sense and are clear to people. Work is being done to enhance what is online and improve the digital communication on the JDRF website. There are also informative videos that are being added. An example is a story of twins with one diagnosed with T1D and the finding that the second twin was positive for autoantibodies. The second twin was diagnosed six years later, but with knowing this information the start of their T1D was still with a very low A1C and very little hyperglycemia. Their mom was also found to have autoantibodies. The new information on the JDRF website will help to reach more people about screening. The efforts JDRF engaged in with paid advertising in April and then in September that discussed the T1Dectect screening program, resulted in this being the number one most visited JDRF site. This shows that we can get people to this information and the work will continue to help people once they get to the T1Detect website. JDRF is working to make sure that they have the material they need to make an informed decision about screening and their healthcare. There are many staff involved with these new efforts at JDRF both on the Clinical Trials/Research side and the JDRF healthcare policy side.
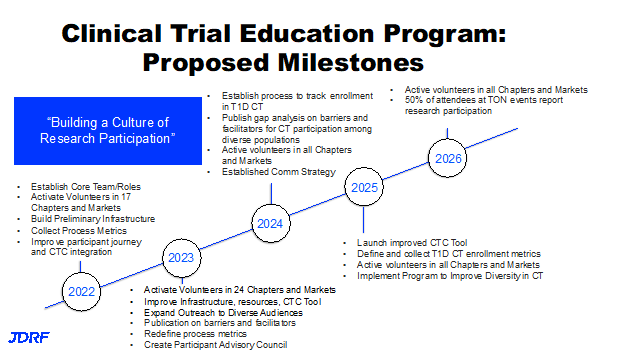
This newer clinical trial education program has the goal to create a culture of research participation in the T1D community. This is to reach all in the T1D community and not just those involved in the JDRF T1D community. The goal is to engage people in research, move science forward at a quicker pace so we can rule things in and rules things out. In this way we can ultimately get to cures and better treatments faster. The clinical trials education has five components. The first is the program called the Clinical Trial Education Volunteers (CTEV). Second is the Clinical Trial Connection Tool. The next parts are education and outreach, with much of these coming online. This includes work on improving the evidence based science and putting this evidence based science behind best practices. Lastly, there is a new participant advisory council. Here are the five components in more detail. The first is the CTEV program. There are now 24 local volunteers and this is growing at a reasonable pace. These local volunteers work in partnership to educate their local community about the importance of clinical trials, what they are, how to enroll, what to expect when you get there. They want to inspire people to learn more about research participation as only small percentage of people participate in research and we need that number to increase. We also need people from all walks of life and a diverse group of participants, because that’s not what we see typically in T1D research at this time. We want to connect individuals and families to local study investigators and let them talk about inclusion criteria and the details of a study. The CTEV volunteers are those with the JDRF banners for clinical trials, they have a table at events, they have QR codes to help you find a trial and explain how to connect with a trial. These volunteers are engaging with monthly pop-up study updates to stay current. This group is fairly small as there is a lot of interaction required between the volunteer staff and the principal investigators along with having access to a clinical trial dashboard. They can download spreadsheets from that dashboard to share those studies that are enrolling locally with people in their community. They also represent JDRF on a number of panels. As an example, one CTEV went to Bethesda, Maryland and represented people living with T1D at a beta cell symposium that was promoted by JDRF and the NIDDK at NIH. There is also an online hub for the CTEV. This hub hosts a lot of resources they can use, they can print off tables, IRB approved study, along with other videos or text that can be shared on social media or through the chapter website and social media. The dashboard also shows the virtual trials that recruit all over the country. This means you don’t have to live in a specific geography to participate and to contribute to research and to
contribute to science. This is a great option as well. The CTEV is the person to get to know in your chapter.
This year these CTEV’s are looking at ways at JDRF events to reach out to underrepresented populations in their market or in their chapter. JDRF is also working with Ashley Butler, PhD at Texas Children’s Hospital, who is a psychologist who works on diversity in clinical trial participation. She is helping to bring her expertise to these events to make sure that we are doing them in an authentic and purposeful way. One additional point is that the clinical trials for most of the prevention studies, provide traveling funding. This means you do not have to necessarily live nearby a clinical trial site to participate, as the costs are sometimes covered. Several years ago, JDRF funded a study to build the evidence base that would help to understand the barriers and facilitators to clinical trial participation in T1D research. JDRF is also focusing on diversity, as we know that people who identify with a racial or ethnic minority often are left out of research. If we do not include everyone in research, we will not have cures for everyone as they are not reflected in the results of a study. We need to make sure that we are identifying unique barriers, and making sure that participation and access is open to everyone. JDRF also looks at the principal investigator’s role in diversity, inclusion, equity and belonging. There are already practices taking place that support diversity and inclusion. JDRF’s work is also informed by what a lot of clinical trial coordinators are already doing. There are educational videos that address several topics. First, we want to make sure people know what a clinical trial is, or a clinical study. We want people to know that they should participate at a level that makes them feel comfortable. If they feel comfortable answering a two-question survey in clinic to contribute to a better process for T1D care, that is great. If they want to participate in a 14-day infusion of a therapeutic, that is also wonderful. Participants can make a choice based on their personal needs, and any concerns they might have. We want people to know about clinical trials. We want them to know why it is so important to participate. Often clinical trials are delayed or do not get completed because they do not have enough people and also those in diverse groups participating. JDRF is working to talk more about prevention and screening. Without prevention, there will not be a cure. Work is being done to have information on prevention and screening in Spanish available this year. It also helps to educate people to advocate for themselves to participate in research, as sometimes their clinics are very busy places. Research doesn’t always get mentioned by healthcare professionals, so it is helpful if people also ask. Again, we need to make sure people know how to participate and know why they should participate and where to make the connection to do so.
The Clinical Trial Connection Tool can give you a clinical trial match within 60 seconds. The connection tool software goes out and pulls trials off of clinicaltrials.gov. It tells you trials that are within 50 or 200 miles or more from you to make it more helpful. When people use this tool, they can opt in to give JDRF their email. It is a box on the bottom left. When JDRF gets your email address, that is the only information they get from that click is the email. JDRF is working to then reach out to give a person more information about clinical trials in their area and ask if they would like to share more of their information for finding the right trial for them. So far over 10,000 people have been interested by sharing their email and a significant number have shared more information also. Currently, JDRF is building a process for the CTEV in your chapter to be able to more easily communicate with the constituents in their chapter. This will really keep the focus on only clinical trial content, and not a lot of other JDRF content at this time, people can also opt in by filling out a form on the JDRF website, not just by using the Clinical Trial Connection Tool.

The Participant Advisory Council is new to JDRF. JDRF gets a lot of requests from study investigators, from startup companies and the T1D therapeutic space, who say, we are designing a study and we would love to talk to someone with T1D, or someone with presymptomatic stage one or stage two T1D, or a parent of a very young child with T1D, to understand what study design would be acceptable, and would it make sense for that individual or parent. This Participant Advisory Council is a group of volunteers to inform study design. This will consist of parents of children with T1D or have children at risk, those that are antibody positive, adults living with T1D who were diagnosed as children or adults, and individuals at risk for T1D and are in stage one or two. The need is to represent all on this council. There should be people form diverse racial and ethnic groups, genders, rural and urban settings, also with different insurance status, public and private insurance. There will be more about all of this in 2023.

Second half of this article has information from Aaron Turner Pfeiffer-JDRF Director of Health Policy
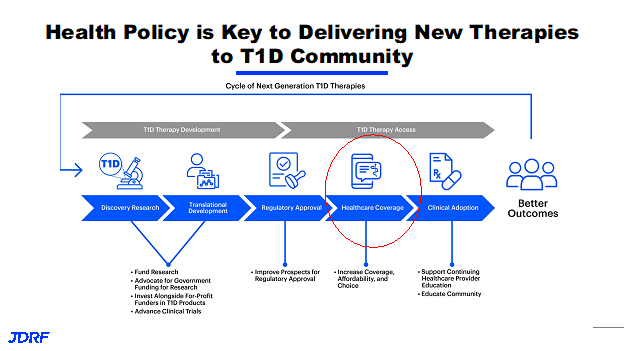
Health policy occurs in the middle of the JDRF pipeline. This is where JDRF has their efforts around regulatory policy and regulatory approval. On the right side in the pipeline is healthcare coverage and clinical adoption. The big red circle is where most of the healthcare policy work is focused and supports the community engagement team on many things around clinical adoption. The work of the policy team is really focused on healthcare coverage. Health policy at JDRF and the health policy team is focused on ensuring that folks have access to innovative therapies that JDRF is funding from the research side and working through moving them through the pipeline. This team makes sure that folks have access, affordable access to those therapies, and also to ensure that folks are getting access to standard of care and are having better outcomes via insurance design, an employer benefit design, and then clinical care and clinical coverage. What that may look like in practice is a number of different things. The health policy team is leading those efforts. We are supporting our colleagues on the advocacy team in different ways in different roles. Health policy issues we approach through a couple of different strategies, tactics, and we are also a resource for the community. There is direct engagement with decision makers which can be folks in government, the White House, Congress, the Department of Health and Human Services and Medicare regulators, but also directly with employers and health plans, other stakeholders and depending on the issue could include manufactures of devices and drugs. This department of JDRF really makes an effort to directly engage decision makers and not just provide resources. They want to make sure that folks are aware of the issues that are happening in the T1D community as well as the impact that those decisions are having. There is a vast and great JDRF grassroots network.
The health policy team provides support for people so they can directly engage Congress, their health plans and employers as all of these things are interconnected. The resources that are provided to the community also are a great tool and resource for the grassroots team. There are also allies such as other advocacy organizations, and where appropriate, possible stakeholders in the industry to advance JDRF goals and mission. There is a great resource that JDRF provides to the T1D community and is maintained and supported by the whole policy team and that is the JDRF health insurance guide. Every year new terms are added to health insurance that makes it more complicated. This will continue to be a good resource to help folks navigate those open enrollments periods and also to help plan and get through some of the barriers that are going to come up in a plan year. This resource is also available on the JDRF website.

It is really important to utilize this and it does a great job of walking folks through some of the common terminology that is often a barrier to understanding some of the dynamics at play within your health insurance. It covers both commercial insurance, Medicare and there are some resources on there highlighting different elements of Medicaid plans as well. The umbrella campaign for all of this is Coverage to Control. This has been part of JDRF’s policy work to provide engagement on a number of different issues.
There are three key elements or three key issues over the last couple of years that the policy team has engaged on. Under the affordability bucket, the focus was around insulin affordability and choices to give folks choice to utilize the insulin pump that works best for them. Work was also on coverage to make sure all can have access to life saving technology, including your artificial pancreas system that is covered by insurers. On the policy side, it is always looking for improved access. It is organized around affordability, choice and coverage. Key areas are addressed that employers, in particular, are interested in for their benefit plans. Employers and others may or may not be providing the best coverage or decisions in their benefit design. The new area that was not covered in Coverage to Control is cures access. This is an emerging area that JDRF will be spending a lot of time on the policy side.
Thanks to JDRF, we now have therapies that have moved through the research pipeline and FDA regulatory approval such as Tzield (Teplizumab) and we are now looking at these therapies and what coverage looks like for these therapies. It is an exciting time for these cure therapies. When looking at cure access, we are talking about screening, monitoring, as well access to the therapies themselves. In some cases, monitoring is the most appropriate path forward for care. When we think of three elements together as we are talking about cures access, the landscape is at best uninformed and unstructured in terms of coverage. This finds screening and monitoring is not part of routine preventative services. There is no clear methodology around how payers are paying for screening and monitoring. There is some emerging dynamics that are starting to reveal themselves as we have the first diseases modifying therapy for T1D to hit the market. In the experience of engaging with payers and the broader provider community, there is limited awareness around screening, monitoring, disease modifying therapies and cell based therapies for T1D. There is a lot of work to do to get where we want to go for the future with broad elevated knowledge amongst all the stakeholders and routine screening for T1D diabetes for everyone.
There needs to be general population screening. There needs to be broad coverage both by commercial payer programs as well as government programs, such as Medicaid and Medicare. There is a lot to do to get there to build an infrastructure, not just the infrastructure around research, but also the infrastructure, working with the regulatory folks on the FDA side to help move these things along. JDRF has done a tremendous job of building that infrastructure out to get us where we are today. The health policy team really picks up the baton, to help build out both the insurance structure and coverage that’s required, as well as the work of Anastasia and the community engagement team to make sure there is a clinical infrastructure to support screening, monitoring and cures therapy access. We think about this in a couple of different ways. Underpinning access to curative therapies, disease modifying therapies and cell based therapies includes broad access to screening and monitoring. We think of these things as foundational elements to ensure that there is access to the therapies themselves when they come online. There are two buckets here, that are going to be mutually supportive, moving at the same time, but potentially under a different time horizon. In the public policy campaign, this clinical adoption, will ensure there is reimbursement and insurance coverage for screening and monitoring. This is occurring now. There is data that JDRF has been supportive of generating, and with others in the community, that have provided for both a clinical foundation. There are clinical guidelines being updated, that highlight the importance of screening and monitoring. We are doing our best to advocate and inform the environment so there is coverage for these. The much broader public health campaign is changing over the long term, the ADA guidelines, including that there are screening and monitoring requirements and pediatric guidelines.
Ultimately, the Preventive Services Task Force will recommend T1D screening and monitoring. This will help to check all of the boxes for the public health campaign. If you can generate enough clinical data that demonstrates the value and importance of screening, get ADA guidelines updated, to the association of pediatricians Bright Futures and the US Preventative Services Task Force recommendations, it will move forward. These elements both either by law or by custom and practice, often trigger automatic coverage and reimbursement through the insurance system. Each of these steps here on the public health campaign have different requirements, different data expectations and they require a little bit more time to prepare and present materials. The time horizon here on the public health side is much longer, but that doesn’t mean that we won’t get there. That doesn’t mean that there is not going to be coverage happening via the existing insurance system and via different pathways in which health plans and government programs will cover different services that have yet to be endorsed by the Preventive Services Task Force, as an example. So those are things that JDRF is building now and it is a very exciting time. This is the start of what will likely be a long journey, but it is already bearing fruit.
Other things that policy continues to work on is the issue of insulin affordability given its importance to our community. This is not just the health policy team, but also our advocacy team is large working on both with government policymakers, they also are working directly with other stakeholders outside of government to make sure we are lowering the cost of insulin. No one should go without insulin because of the cost. Our efforts are focused on that and a couple of the areas where we’ve really driven and tried to educate on decision makers, is around the impact with the rebate system. Designing your benefit plan has a huge impact on someone with diabetes and the level of education and knowledge, especially amongst employers and HR professionals, who are making these decisions on behalf of people they employ with diabetes. We’ve been focused on the last couple of years and with these efforts it has bared fruit. There have been good strides in progress around improving benefit design, that we are happy with, but we know we have a long way to go. We are happy with the progress also and continue to try where we can provide resources. There are a number of affordability programs that are being offered either by other not for profits or by the insulin manufacturers themselves. We are always a resource for folks who are in need.
JDRF is constantly working to have the stakeholders in the drug supply chain, do their part to lower insulin costs. We are not just focused on Federal legislation that affects Federal policy changes, but we are also directly engaging insulin manufacturers, health plans and employers to do their part to make these changes as well. There was progress in the Inflation Reduction Act, it took an important step certainly but did not go far enough. JDRF is continuing to work for legislation that will provide relief for the top line insulin prices, as well as cost protections for the commercial population. The Inflation Reduction Act did cap insulin costs out of pocket at $35 a month for folks enrolled in Medicare. We have seen over the last year, more studies, more data points coming out showing it actually makes zero sense to charge someone out of pocket cost for life saving medication. Often, insulin is the example used using these data points. We see more insurers and more pharmacy benefits managers (PBM’s) moving in the right direction. For most populations, we’ve seen that manufacturers enhance their assistance programs and certainly do not want to be in a situation where we have to ask or expect manufacturers to provide assistance because their insulin is so high priced. These programs are certainly a lifeline for many and some important elements continue to be offered. We have seen some manufacturers offering both high price branded products and a lower price branded product. This is not ideal, but access to a lower price insulin is again a lifeline for millions. It is an important element that continues to be offered and an important development in terms of manufacturers business strategies. The last point, is the partnership with Civica. This in indicative of JDRF efforts around insulin affordability.

There has been progress made in many ways. This also highlights the work that the T1D community has done at large raising this issue at a time when folks, especially in government work are paying attention and talking about the impact of high insulin prices on people with diabetes. It is the number one example of the broken system and the impact of a sorting system that rewards higher list prices. The impact that has on the people who take those drugs and insulin in the number one example. JDRF has been at a couple of events over the last couple of months at the White House where insulin and T1D has been a focus mentioned by the President and this is a bipartisan concern. There was support for lower insulin prices from the previous administration as well. This is a testament to JDRF’s work and the work of the large T1D community that we are at a point now where we are getting awareness and attention on this issue and are seeing small but steady improvements and the availability of low cost insulin.
The work to lower insulin pricing is really an organizational effort and an advocacy effort to try and approach this differently. Many have seen the JDRF announcement supporting and partnering with Civica and other leading healthcare stakeholders to try and disrupt the insulin market. There is steady progress being made, but not enough is being done to truly lower the list price. This is an issue that needs attention now. That is why JDRF is partnering with Civica to offer the lowest priced insulin available on the market at $30 per vial and $35 for a box of pens and this will really cut out and get around the distorted effort that rebates are having currently.
Not only will this provide a low cost insulin for millions, it will provide some peace of mind. If you are getting insulin by mail and it doesn’t make it in time, you don’t have to fear having to go and pay out of pocket at a cash price of $400 for a vial of insulin to bridge you through until your next shipment arrives. You are going to know, a low cost insulin from Civica is available for $30 a vial. JDRF is very excited about this opportunity. The Civica team is making good progress and good strides to make sure the first insulin is available in 2024. This highlights that this is an area where we are not waiting for government action or health plan action to lower insulin prices, JDRF is doing this to provide relief as quick as possible.
In the Coverage to Control campaign the focus for JDRF was making sure there was coverage and that people had a choice. In a large part, we have achieved what we set out to achieve when we launched this campaign as related to devices a couple of years ago. Commercial insurers by and large now cover CGM and they cover artificial pancreas systems. By and large, they cover all the approved insulin pumps. In recent changes, Medicare has caught up to the commercial side and they are covering insulin pumps and CGM’s that are available on the market. We have made tremendous strides and JDRF really has been the driving force behind this for the last decade of getting CGM accepted, coverage on the commercial side and the driving force getting Medicare to catch up to standards of care and cover CGM and artificial pancreas systems for those rolling into Medicare. Unfortunately, Medicaid is still lagging behind the commercial Medicare side, but there are leading states that are now covering CGM and artificial pancreas systems for kids. We have reached the phase now with insurance policy both on the government side and on the commercial side where they are no longer questioning the value of CGM and insulin pumps. We are to the point now that the American Insurance system is covering all devices and are trying to pick their favorite devices, which this is creating issues for folks and unnecessarily, forcing folks to change devices that otherwise would work for them. Currently, JDRF continues to work through the machinations of the American system. Questions will arise for the next generation of devices such as how is coverage interacting or impacting devices where there’s now interaction with a cell phone and an algorithm. There will be unique challenges that are starting to present themselves and we know from the commercial side, as the devices are moving from a durable medical equipment benefit to a drug formulary, there might be some challenges associated with that.
Health policy is paying close attention to these changes. Medicare is unique in that once you got something covered, you can kind of put that issue to rest because they rarely go back and change something. They are also slow to adapt to innovation. We know that the device space is rapidly innovating, with interactions with cell phones and algorithms. That is going to be a tweak of how Medicare covers that issue. We are still expecting Medicaid to catch up to where commercial insurers have been and where Medicare is today. We are still working with our allied partners to see what we can do to speed Medicare and Medicaid coverage. It is a great time to actually be looking at device access and we have come a long way. JDRF is proud of the role it has played in helping get device access and coverage. The Health Policy Team and Advocacy has an integral role in accelerating affordable access to innovative and life improving therapies and we do that through direct engagement with stakeholders and policymakers. We are really a resource also for the T1D community and you should check out the JDRF insurance guide and we encourage you to share it with others. Our team is also a resource for all staff volunteers in this community. Also, JDRF does want to hear from its community. This is one of the ways that we stay informed and that is by folks sharing intel that they are aware of that is happening out there. This helps JDRF keep a good finger on the pulse of what’s happening in terms of insurance changes that are impacting the T1D community.
Please reach out to me, Debbie Evans, CTEV for the JDRF Minnesota and Dakotas chapter, if you have any questions about clinical trials, screening and policy questions. If there is something that you ask that needs a deeper dive, I also know who has that information. I can be reached at debbieaevans1@gmail.com .