Portland, Spokane & Seattle – DR:EAM Study for Diabetic Retinopathy

For people living with type 1 diabetes (T1D), external doses of insulin save their lives—every day. Yet keeping blood-sugar levels within a healthy range remains a daily challenge, putting them at risk of both short- and long-term complications. JDRF is committed to making the management of T1D much better and safer through research and clinical trials to develop new therapies that can help keep your blood-sugar levels in range, reduce insulin dosage, and decrease complications that come from the disease.
One very common long-term complication of T1D is diabetic retinopathy. Diabetic retinopathy (DR) occurs when the blood vessels at the back of the eye (the retina) are damaged. If left undiagnosed and untreated, DR can lead to visual problems and even blindness. The retina is the part of the eye that senses light. It sends electrical signals to the brain to interpret what is seen and creates an image. People with DR may have a loss of central vision, blurred vision, or other visual disturbances (floaters or black spots).
The majority of people with DR are of working age (18 to 65 years). As such, approved treatments that have minimal side effects and do not have a big impact on daily activities are highly desirable. The current standard of care involves injections into the eye once patients develop severe disease and/or experience vision loss. The DR:EAM Study is looking at a less invasive approach with an eye drop that can be used at an earlier stage of DR. This is a multi-site study, including participating sites in Portland, Spokane and Seattle.
THE STUDY:
Aim
In the DR:EAM Study, the use of an investigational topical eye drop will be assessed in men and women with type 1 or 2 diabetes mellitus who also have DR and who have not received any previous treatment for their DR.
Participants
Around 210 men and women in the United States are expected to take part in the DR:EAM Study.
Inclusion Criteria
To take part in the DR:EAM Study, you must:
- be 18 years of age or older
- have been diagnosed with type 1 or 2 diabetes mellitus
- have DR
- not have received previous treatment for DR
The Investigational Drug
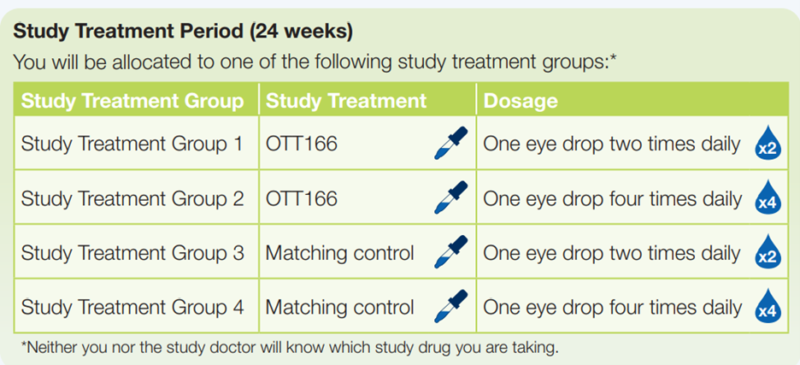
The investigational drug is called OTT166. It is described as investigational because it has not been approved to be prescribed by doctors and other medical professionals for DR and is still being researched in clinical studies like this one. In the DR:EAM Study, participants will be asked to self-administer a single eye drop of the study drug (OTT166 or matching control) every day, two or four times a day, for 24 weeks. The control looks like OTT166 but contains no active drug.
Study Design
The DR:EAM Study is made up of the following periods:
Screening Period
- Up to 3 weeks before the study starts, you will be invited to the study center to have some tests to check you are able to join the study
Study Treatment Period (24 weeks)
- You will be asked to self-administer the allocated study drug every day at the prescribed frequency
- You will be required to visit the study center around once a month (8 visits total) to have tests/assessments. These may include:
- Eye exams
- A physical examination
- Checking of your vital signs (blood pressure and heartbeat)
- Taking samples of your blood and urine
Contact Information
If you would like to learn more about the DR:EAM Study, please contact:
- Portland
- Site: OHSU Casey Eye Institute
- Contact: Ann Lundquist (Study Coordinator), lundquia@ohsu.edu, 503-494-6364
- Spokane
- Site: Spokane Eye Clinical Research
- Contact: Dylan Waidelich (Director of Clinical Research), research@spokaneeye.com, 509-623-9768
- Seattle
- Site: Northwest Eye Surgeons at Seattle Northgate Clinic
- Contact: Hannah Seo (Clinical Research Director), 206-528-6000 x3815, online submission form at nweyes.com/clinical-trials under “Retina”
Contacting a site does not mean that you must join the study or that you will be able to participate. The participating sites will be happy to provide you with the information you need to make an informed decision.