JDRF Research First Hand – Clinical Trial Connections
Participating in a clinical trial is a great way to contribute to curing, preventing and treating T1D and its complications. About 20,000 people are needed for T1D trials in the U.S., but researchers report a massive shortage of participants. In fact, more than 80% of clinical trials are delayed or fail because doctors cannot find enough patients to participate. Additionally, clinical trials provide an opportunity to access new medical advances; in some cases, participants are able to continue therapy after the end of the trial if it’s found to be effective. Participants also receive free, top quality care and appreciate being able to help move research forward. For more information about participating in a clinical trial near you, visit the JDRF Clinical Trials Connection.
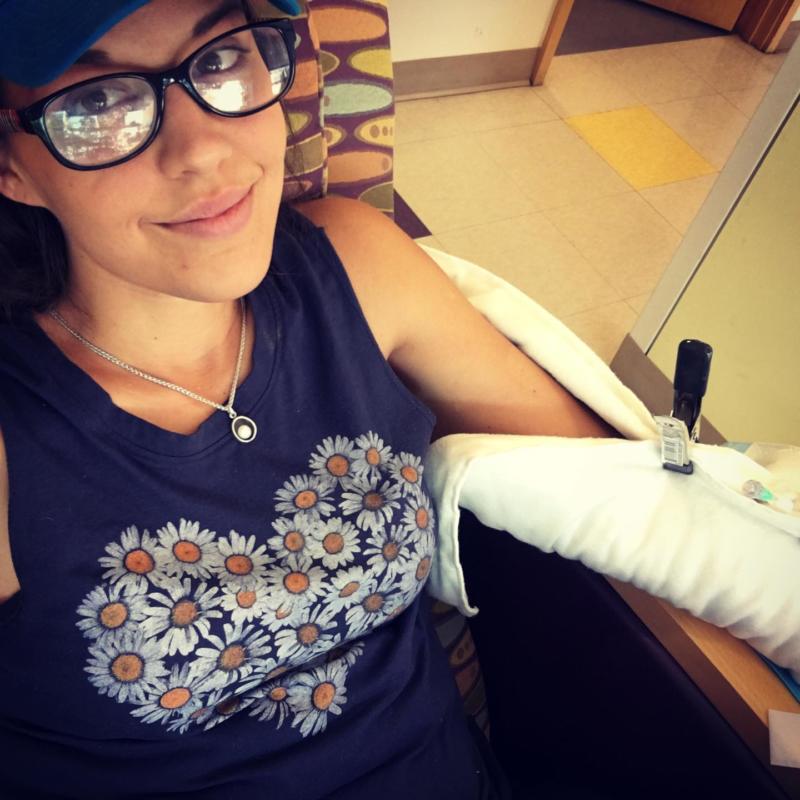
JDRF Outreach Manager Kelli Raleigh is in the midst of the Colorado-based clinical trial Senseonics Promise Study, and shares a first-hand account of her experience.
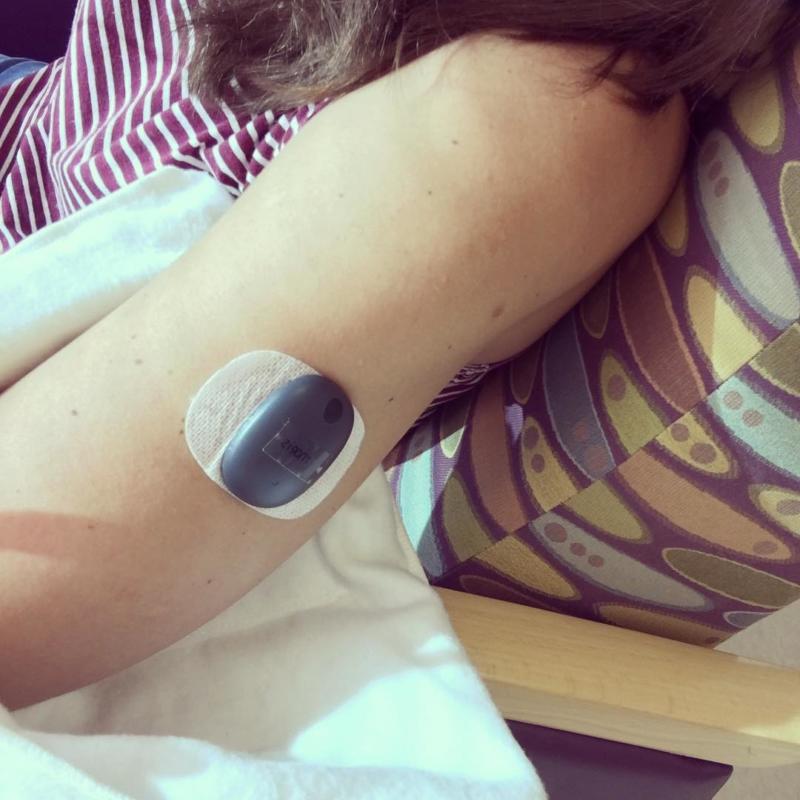
For this trial I’m allowed to wear both my Dexcom CGM and the Eversense by Senseonics and frequently compare data. They’re both accurate and Eversense doesn’t seem to have the slight lag time other CGMs that sit in the interstitial fluid tend to have. Eversense uses a little light membrane to measure glucose in the blood and that little change in technology seems to make a difference. I’ve also not experienced any compression lows like I do with my other CGM when I sleep.
I think I’m most impressed with the idea that you never have to worry about your sensor ripping out or coming off like you do with other CGMs. Each day you change out the adhesive patch that the transmitter is stuck to and voila – that’s it! No pokes, needles, or anything needed. Additionally – if you want to just take it off for an evening or for an outfit when you don’t want your transmitter to show – you can. No evidence of CGM unless, like me, you have a propensity to develop tan lines quickly.
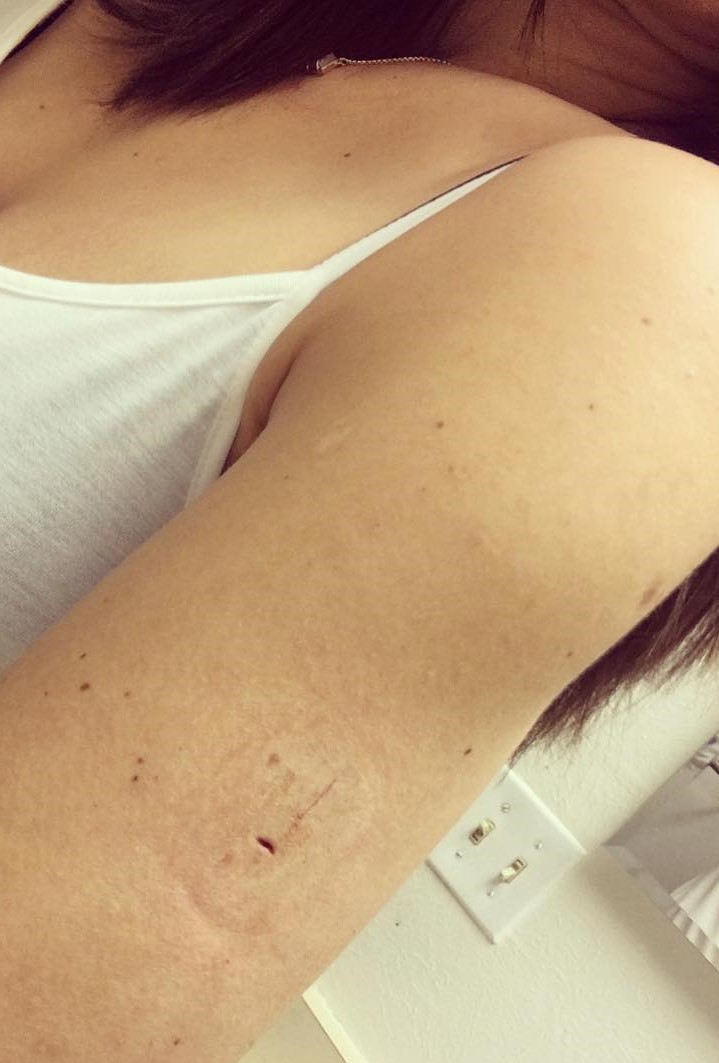
I was most worried about scarring going into the trial – I was certain I’d have a bump scar but, much to my surprise, you can’t even see the incision spot unless you really look after 90 days.
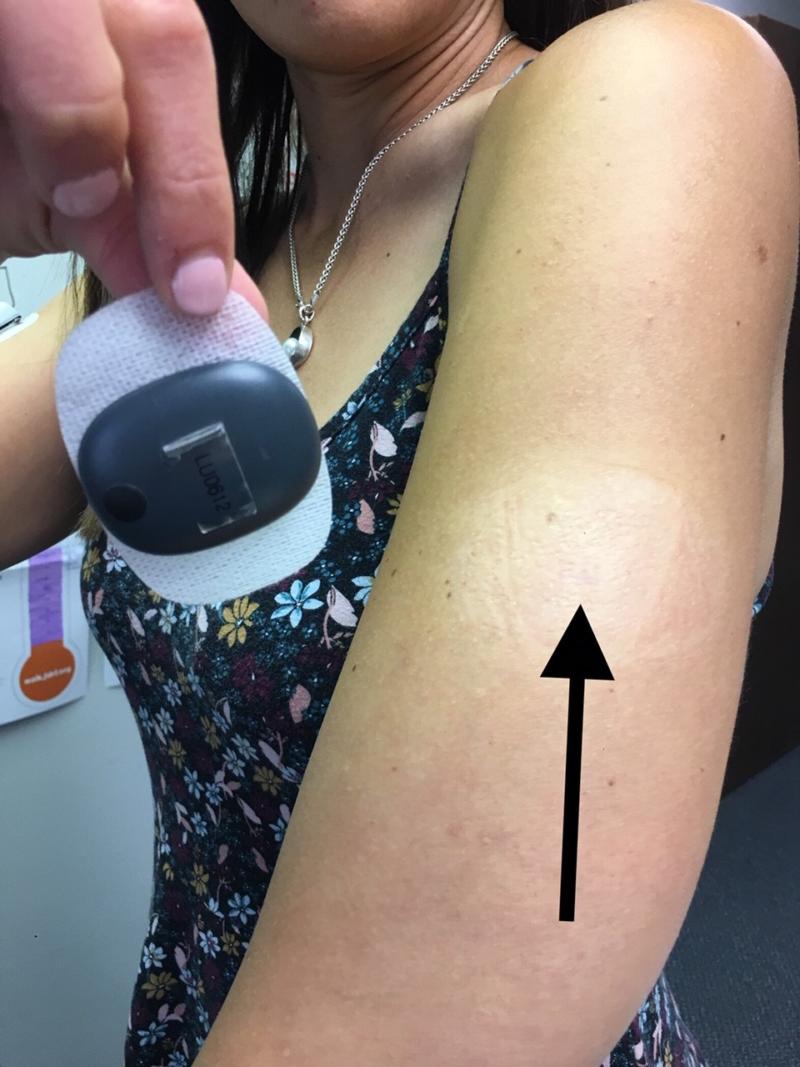
I share clinical trial updates on my Instagram page frequently – you can follow me on IG: @ Kelli_roo for more updates and to ask me any questions you might have!
Besides impacting research that will potentially help millions living with or at risk for T1D, the benefits of clinical trials may include:
- Getting access to the latest technology or medicine in T1D research;
- Study medications and devices that are provided to you at no charge;
- Being reimbursed for your time;
- Meeting cool people
- Receiving more frequent care and checkups by the study care team
JDRF has made is easy to find clinical trials for any age and diagnosis state. In fact, when I used the tool it revealed more than 100 trials near me. Though I did not have T1D at the time, I was 8 years old when I did my first clinical trial. I’m now 36, have lived with T1D for 18 years, and have participated in several clinical trials.