Coating on Capsules Next Step in Creating Insulin-Producing Factories

One potential cure for type 1 diabetes (T1D) is to replace beta cells destroyed by a person’s own immune system with healthy beta cells inside a device, which would then be placed inside the person’s body. Encapsulated cells would release insulin in response to changes in glucose levels. That is exactly the scenario that several JDRF-funded researchers have been working on for several years, including a team of bioengineers at the Massachusetts Institute of Technology (MIT). And recently, the researchers have moved one step closer.
A key challenge for the clinical use of implanted biomaterials, mostly cell encapsulation devices, is the deposition of scar tissue around the device that suffocates the cells and prevents long-term function. This process is called fibrosis, and occurs when a person’s immune system attacks foreign objects in the body such as the capsule with beta cells inside. The team has extensive experience designing “smart” biomaterials for clinical use. In a previous study, the team coated the device with a small-molecule drug called THPT. That particular drug blocks the development of scar tissue when implanted in laboratory animals.
In the latest Nature Biomedical Engineering journal study, the team found that wrapping the implantable device with a synthetic polymer coating with openings, or pores, of a particular size enables small molecules—like nutrients and oxygen—to pass through the coating into the capsule and nourish the beta cells The pores even allow certain immune cells like macrophages, which act as vacuum cleaners for foreign substances, to get inside the capsule. But at the same time larger T cells, the body’s immune cells that attack beta cells in people with T1D, were kept at bay.
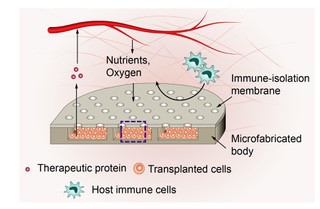
These are critical discoveries. People living with T1D that receive transplanted beta cells have to take drugs that suppress their immune system, so that T cells don’t destroy the new beta cells. However, those drugs can have serious side effects, and make people more vulnerable to infections.
“Recent advances have made it possible to generate an essentially limitless supply of human, glucose-responsive insulin-secreting beta cells from stem cells. The challenge is how to use these in patients without requiring systemic immune suppression”, says Daniel Anderson, Ph.D., an associate professor of chemical engineering at MIT. “This device allows us to transplant these cells to survive and function without immune suppression, thereby providing blood sugar control without outside injected insulin.”
Previously, the researchers had developed the silicon-based capsule itself. Then they worked on the coating, which is thin enough to wrap around the capsule or even a person’s organs. It is about as stiff as a piece of tissue paper.
Without THPT, fibrosis forms around the capsule much like a callus on a person’s foot. That extra scar tissue makes it nearly impossible for blood to bring nutrients and oxygen to the islet cells to keep them functioning correctly. Dr. Anderson, Robert Langer, Sc.D., José Oberholzer, M.D., and Omid Veiseh, Ph.D. all JDRF-funded researchers, founded Sigilon Therapeutics, which has patented the use of the THPT coating for implantable devices and is now developing treatments based on this approach.
The researchers were able to show in experimental models that transplanted beta cells maintained normal blood glucose levels for more than 10 weeks in diabetic animals. The team continues to work on improving the device to lengthen the time that it can control blood sugar levels. “We think carefully engineering the interface between the device and the body, as well as the positioning of the cells within the device, will be needed to ensure the insulin-producing cells receive appropriate levels of nutrition and oxygen,” said Dr. Anderson. “It is our hope that this is the key step needed to ensure long term function.” The team will next work on the placement of the device in the body, as well as where the beta cells are placed in the device, to improve the length of time that the device works.
This series of advancements shows just how much impact JDRF donors make toward cures for T1D.
If you can at this time, please consider supporting JDRF once again. Together, we deliver life-changing breakthroughs for people with this condition. Please make a gift today.