FDA-Approved Immune Therapy Shows Benefit in Newly Diagnosed
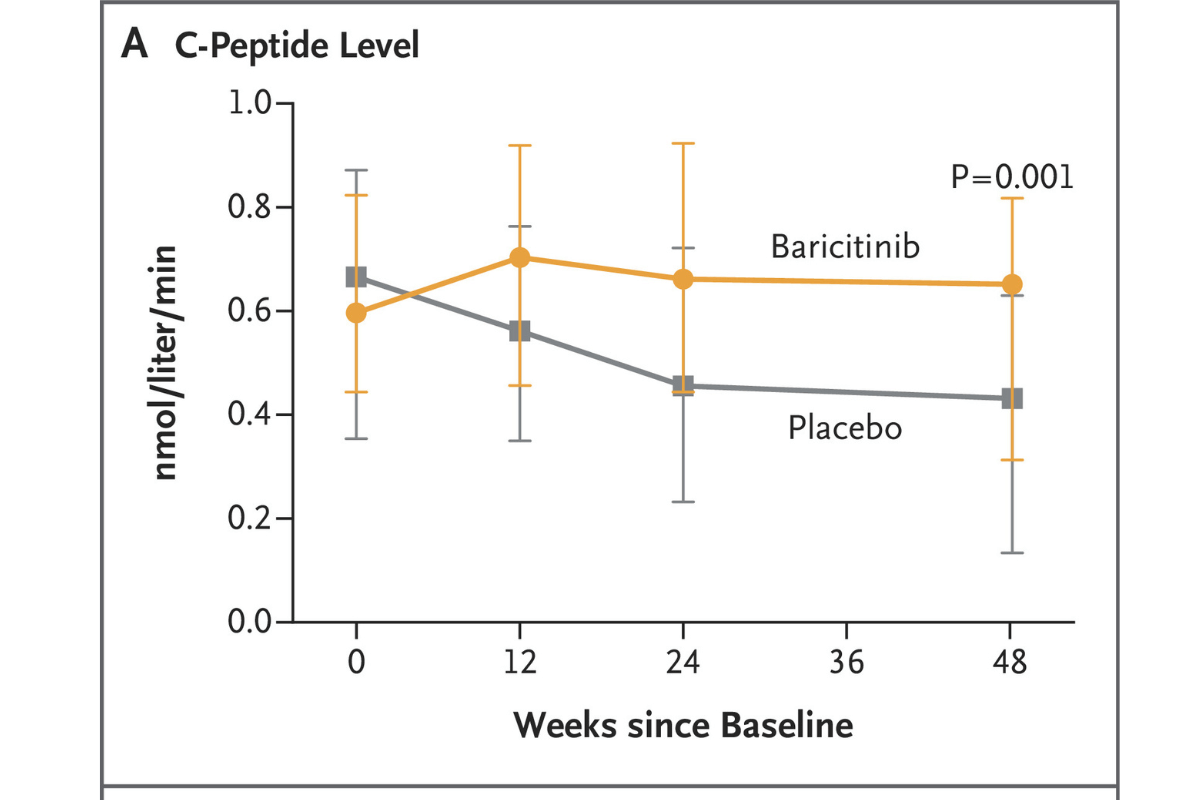
In a JDRF-funded clinical trial, published in the renowned New England Journal of Medicine, Thomas Kay, M.B.B.S., Helen Thomas, Ph.D., and others demonstrated that baricitinib—a JAK inhibitor, which is critical to signaling pathways within both immune cells and beta cells in type 1 diabetes (T1D)—preserved beta cell function in the disease.
In 60 newly diagnosed children and young adults, baricitinib:
- Preserved insulin production, as estimated by C-peptide
- Improved blood-sugar variability and time-in-range, using a continuous glucose monitor (CGM)
- Decreased the requirement for external insulin
- Was well tolerated
The effect of baricitinib was achieved using a single daily oral tablet, and it’s the first immunotherapy trial to suggest a benefit on CGM measures. (Verapamil, a once-a-day tablet, also preserved beta cell function, but without improvement in CGM measures or insulin requirement.)
What Comes Next?
Baricitinib is not an FDA-approved therapy for people with T1D, but JDRF has multiple lines of inquiry to make sure that this and other disease-modifying drugs get to the hands of people with the disease. There are several clinical trials that JDRF is exploring to see if baricitinib can be effective if used in conjunction with other therapies, such as Tzield™ (teplizumab-mzwv) or verapamil, in presymptomatic disease, or in longer duration.
But this adds to the armamentarium of potential curative therapies, and JDRF is excited to be a part of the team that made this advance possible.