2021 JDRF Advances

June Chapter study sheet – Clinical Trials
JDRF Clinical Trial Connection Tool
JDRF had made amazing progress in 2021 – even the pandemic could not stop us, though it was indeed a difficult year for research because of the pandemic. JDRF’s mission is a world without T1D. The mission is also to improve lives today and tomorrow by accelerating life changing breakthroughs to cure, prevent, and treat T1D and its complications. JDRF is focused on two major areas, which are curing T1D and improving lives. In this year’s portfolio for curing T1D, JDRF has focused on global universal screening, disease modifying therapies (DMT) such as regrowing beta cells and immune therapies, and cell therapies such as beta cell replacement that consists of adding new beta cells from an outside source. JDRF’s Improving Lives program is focusing on better, smaller, less intrusive devices; advanced insulins and adjunctive therapies; therapies for complications and the psychosocial burden of T1D; and bringing better glucose control to patients.
JDRF is working with all stages of T1D. The knowledge of the staging of the disease did not exist 7 or 8 years ago. It is now known that it is a progressive disease that starts often years before a clinical diagnosis.
Stage 1: Normal blood sugar but multiple autoantibodies
Stage 2: Abnormal blood sugar with multiple autoantibodies
Stage 3: Insulin dependance with a clinical diagnosis of T1D with some insulin production occurring
Stage 1 and 2 are part of the disease continuum that is occurring but is asymptomatic.
Established T1D, possibly considered stage 4, is where there is negligible production of
insulin.
CURING T1D
JDRF launched the T1Detect kit and program to help accelerate its mission. This enables not only the families who know they have a T1D connection, but also offers the general population the opportunity to test for the autoantibodies that cause T1D. Of those that are diagnosed with T1D, around 90% are unaware that they had a connection to T1D or other autoimmune diseases. This is the reason to include the general population. About 50% of the new diagnoses are adults. In order to stop the development of T1D, you need to know you have developed these autoantibodies. The goal of JDRF is to accelerate DMT through the pipeline and to improve health outcomes and prevent diabetic ketoacidosis at diagnosis. One of the DMTs that is currently awaiting FDA approval is the ProventionBio drug, teplizumab. This anti-CD3 antibody, teplizumab, has been shown to prevent T1D from developing in at-risk individuals for an average of 3.5 years. These study participants continue to be followed. JDRF’s T1D Fund invested in ProventionBio, which is developing teplizumab and submitting it for FDA approval. JDRF started the research path for teplizumab over a decade ago by funding Dr. Kevan Herold, who made the discovery that this anti-CD3 drug may be able to delay the onset of T1D. The hope is that current clinical studies can show that this drug can also help those that are newly diagnosed with clinical T1D and can help revert back to a prediabetic stage. Support for clinical trials on teplizumab is also provided by the SDP funding that goes for the TrialNet. https://www.trialnet.org
INNODIA is a public-private partnership against T1D in Europe that is co-funded by JDRF. Its goal is to prevent and cure T1D. The INNODIA-JDRF plan is to begin clinical trials testing four different therapies and their combinations. This includes Ver-A-T1D, MELD-ATG, CFZ533 (iscalimab), and IMCY0098 (IMPACT). JDRF is a founding member of INNODIA. Research using verapamil is a continuation of JDRF-funded research, which discovered verapamil’s ability to preserve beta cell function. Through this partnership we have launched 4 therapies in Europe that can change the course of the disease and will now be tested in combinations.
The BANDIT clinical trial is testing baricitinib, a JAK inhibitor (JAKi), in the newly diagnosed. JDRF has supported research into JAKi for more than a decade and worked with Eli Lily to supply the drug for the clinical trial. A JAKi affects both the beta cells and the immunity pathways that affect the beta cells. This trial is currently running in Australia. This drug may be a game changer; there are currently two case studies that saw T1D reversed with these drugs. The results of this trial maybe known in 2 to 3 years.
NEW STUDY FOR ENCAPSULATION
The company Vertex has launched a clinical trial for VX-880, which is a stem cell-derived beta cell therapy for T1D that has received a fast-track designation from the FDA. A phase 1/2 clinical trial has begun in people with T1D with severe hypoglycemia. JDRF funded Douglas Melton at Harvard University starting in 2000 to develop beta cells from stem cells, which he accomplished in 2014. He developed beta cells in a petri dish that could provide enough cells to treat T1D. The JDRF T1D fund invested in his company, Semma. Vertex acquired Semma to develop this technology in 2019. The VX-880-101 Vertex Study is to evaluate safety, tolerability, and efficacy in subjects who have type 1 diabetes mellitus with impaired hypoglycemia awareness. The primary objective is to provide replacement cells for the ones that have been lost or do not work properly in people with diabetes. This is a five-year study with long term follow-up. This study will include participants between the 18-65 years with T1DM. The study endpoints are safety and tolerability, a proportion of participants free of severe hypoglycemia events either with HbA1c <7.0 or a ≥ 1% reduction in HbA1c from baseline. Secondary outcomes are a proportion of participants who are insulin dependent and changes in stimulated C-peptide.
Study Eligibility:
VX880 Study Eligibility
*Male or female between the ages of 18 and 65 (inclusive)
*Clinical history of T1D with >5 years of duration
*At least two episodes of documented severe hypoglycemia in the 12 moths prior to enrollment
*Blood type of A or AB
*Consistent use of CGM for at least three months before screening and willingness to use CGM for the duration of the study
*Do not have advanced complications associated with diabetes including untreated proliferative retinopathy, skin ulcers, or amputations attributable to diabetes
*Willing and able to comply with the study instructions
Vertex is working with a partner to help those who are interested in being part of this study to travel to a study site for the initial part of this study. Follow up visits can be arranged for where you live.
Here is the link to this study: https://www.t1dstudy.com.
The ViaCyte company in San Diego is also working with encapsulation with their own proprietary line on beta cells. Preliminary data from ViaCyte shows that its PEC-Direct therapy helps people with T1D produce insulin again. Last October they showed the cells worked by assaying C-peptide. JDRF underwrote development of the proprietary line of precursor stem cells for their treatment and funded the preclinical and clinical studies of ViaCytes PEC-01 therapies.
Here is the link to ViaCyte’s study being done at the University of Minnesota:
Sernova Corporation, based in Canada, is working on a membrane that will protect the cells from the immune system. Sernova was able to show in a JDRF-funded clinical trial that its cell pouch system can produce insulin in people with T1D. Sernova’s next steps include the development of unlimited locally immune protected insulin-producing cells. They were able to show that this was going to work by first using cadaveric islet cells in their device.
IMPROVING LIVES 2021
Tidepool Loop, an automated insulin delivery app, has been submitted to the FDA for approval. If approved, it will be the second FDA-approved interoperable automated glycemic controller. The app is intended to be used in the future with pumps by Omnipod and Medtronic, along with continuous glucose monitors by Dexcom and Medtronic. Tidepool has been funded by JDRF and the Helmsley Charitable Trust. JDRF founded the Artificial Pancreas Project over 15 years ago to build a thriving ecosystems of AP systems in the market. JDRF funded over $100M in AP research in addition to collaborating with regulatory agencies to accelerate approval. Many use devices already; Tidepool is beyond that and will give an additional choice and interoperability. JDRF played a critical role in this and the AP consortium. Supporting this research has given us these advancements in technology.
Two AP systems, the Medtronic 770g and the Tandem Control IQ, were approved for use in pediatric patients. Approvals have been going down in age and have happened quickly – 24 months after adult approval. Medtronic is now approved for ages 2-6 and Tandem is now approved for ages 6 and up. The next generation Medtronic 780G is approved in Europe but has not yet been approved in the United States. To date, these are the only two FDA-approved AP systems on the market. Both have shown significant benefits to children in clinical trials. Tandem’s original algorithm was developed by JDRF-funded investigators in the AP consortium. Tandem’s clinical trial was supported by the NIH via the Special Diabetes Program.
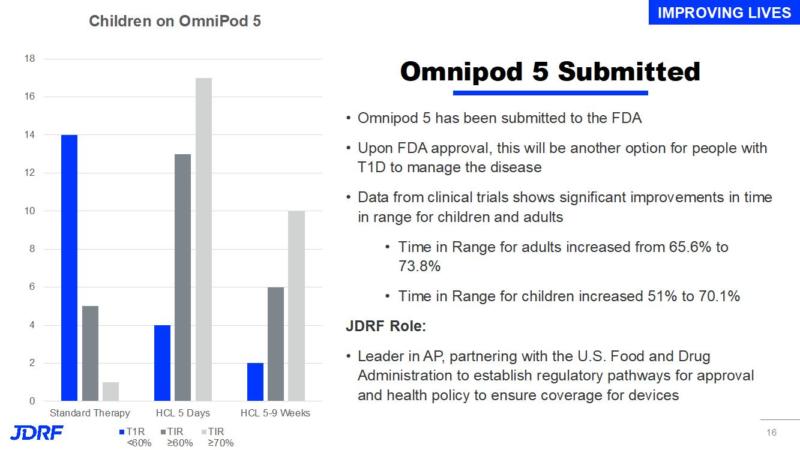
Omnipod 5 has been submitted to the FDA. Upon FDA approval this will be another option for people with T1D to manage their disease. Data from clinical trials shows significant improvements to time in range for children and adults. JDRF has been a leader in AP and by partnering with the FDA has helped establish regulatory pathways for approval and health policy to ensure coverage for devices
*Time in Range for adults increased from 65.65 to 73.8%
*Time in Range for children increased from 51% to 70.1%
ADJUNCT THERAPIES
vTv Therapeutics received fast track designation from the FDA for TTP399 as an adjunctive therapy to insulin. TTP399 is a novel, oral, investigational once-daily glucokinase activator. The results from a phase 2 trial show that TTP399 provided statistically significant improvements in HbA1c without adverse effects, such as increases in ketones or hypoglycemia. JDRF has funded and continues to support the trial and ongoing data analysis. JDRF is working with vTv to move this product through Phase 3 clinical trials. Adjunct therapies used in addition to insulin to improve glucose control and metabolic control can improve quality of life. Insulin is not the only hormone missing in T1D, as there are other imbalances. This glucokinase activator, an enzyme, can help the liver to break down glucose faster.
JDRF is improving outcomes with diabetic retinopathy by supporting a definitive clinical trial testing fenofibrate in both T1D and T2D. Fenofibrate, a VEGV inhibitor, is an oral drug that can prevent diabetic retinopathy from getting worse. JDRF’s role is continuing to support complications research and remains a priority for JDRF. This is just one of several diabetic retinopathy projects that are part of the JDRF Moonshot initiative. The goal is to prevent progression of vision loss and restore vision already lost in diabetic retinopathy. The goal is to test VEGF inhibitors earlier in the disease and determine if earlier and less frequent use of a VEGF inhibitor will prevent onset of macular edema and proliferative diabetic retinopathy. Fenofibrate is oral – not an injection – into the eye. We launched this trial in February of this year. The hope is that is shows that it will help with loss of vision. JDRF has partnered with the Mary Tyler Moore and the S. Robert Levine, MD Charitable Foundation.
There has been a significant amount of progress on complications in the last 30 years, but we still do have complications occur. The reason is, in part, that complications are tied to glucose control and it is still challenging for a T1D to have tight control. In the United States the average HbA1c is still over 8.0, which can lead to complications.
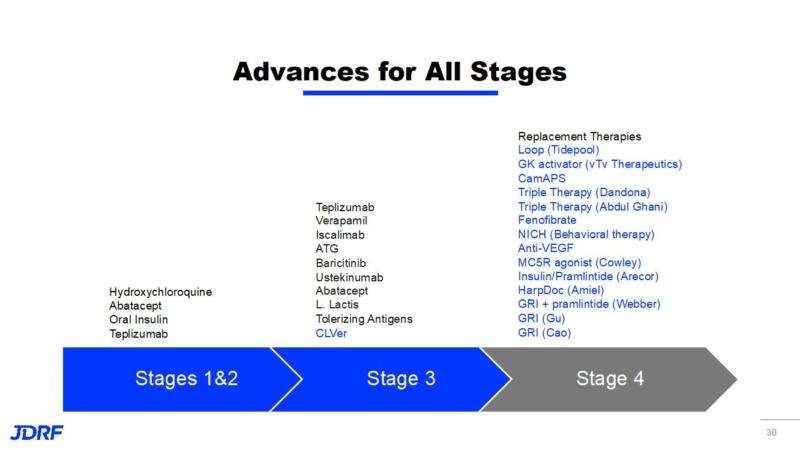
The chart below shows advances and current research in both cure therapies and in improving lives through research on complications. Prevention and cure therapies are being tested in Stages 1 – 3. Stage 4 prioritizes improving lives through better management and reducing complications.
Even during the pandemic, JDRF has been able to successfully fund $506M in total research support. JDRF is able to leverage and bring additional support for funding for T1D research and therapy development. With the JDRF funding of $111.5M in total research support, JDRF funding has encouraged an additional $394M from other partners to equal $506M. For every JDRF research dollar, we bring in another $3.50 to the T1D research and therapy development field.
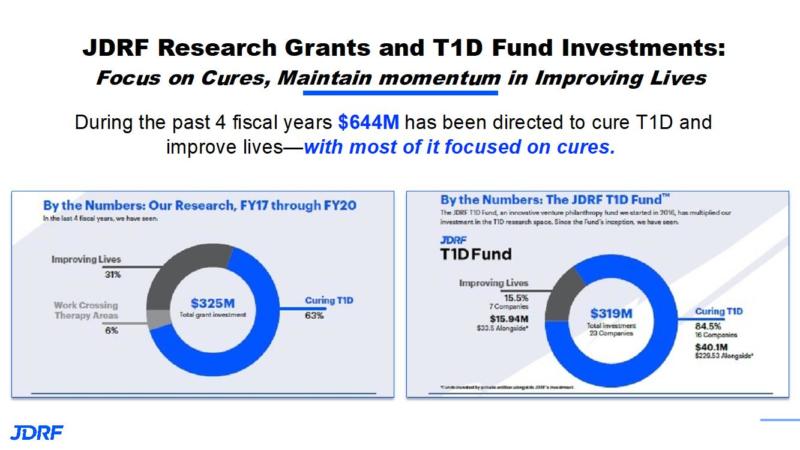
During the past 4 fiscal years $644M has been directed to cure T1D and improve lives, with the major focus being on cure therapies. The JDRF research funding from FY17 to FY20 has been $325M with 31% being on improving lives, 6% on work that crosses therapy areas, and 63% being on curing T1D. The JDRF T1D fund has provided $319M with 15.9% being improving lives an 84% being on curing T1D.
JDRF continues to prioritize clinical trials. In surveys the number one reason people have given for not participating in clinical trials is that they did not know about the trial. We are still in over 70 clinical trials. In every stage of the disease, JDRF is actively funding and having treatments in development. JDRF covers the entire pipeline and basic research is still very important. Also, last year JDRF created the Clinical Trial Education Volunteer (CTEV) program to spread awareness about clinical trials and ensure trials are enrolled faster so they can proceed. This leads to newer therapies getting to patients faster.
This article is a summary of many of the top advances for JDRF in 2021. For more detail on a topic, please see my prior research articles on the specific topic or go to the JDRF YouTube channel and listen to the talk on the topic you are interested in learning more about: https://www.youtube.com/user/jdrfonline.
If you have any questions about the research articles or clinical trials you see on the JDRF Minnesota and Dakotas website, I am the CTEV for our chapter and am helping to develop this program across the country. You can reach me, Debbie Evans, at debbieaevans1@gmail.com or at 612-810-1933. If you call my cell, please leave a message.