Clinical Trials and Disease Modifying Therapies
Use the JDRF MATCHING TOOL to find out what clinical trial is best suited for you!
DISEASE MODIFYING THERAPIES 1 AND 2
This article is a recap of the RIV talk from November and December 2023 on JDRF’s work that is being done on Disease Modifying Therapies. In this talk JDRF scientists, Drs. Joshua Vieth, PhD, Cassandra Bazile, PhD, and Jay Tinklepaugh, PhD, highlight JDRF’s strategy and efforts in this area with a focus on regulating the immune system and beta cell regeneration.
There is a lot of exciting information on how the disease modifying therapy (DMT) JDRF portfolio is moving research forward through the JDRF research pipeline. JDRF starts with funding discovery research that then leads to translational development. JDRF funds research and clinical trials, advocates for government funding for T1D research, and invests alongside for-profit funders to enable us to get to end products. The development section of this pipeline is where this occurs and where ideas are turned into products that eventually go into clinical testing. The next stage is regulatory approval, where we improve the prospects for regulatory by using our advocacy team that is based in Washington, DC. They oversee our healthcare coverage and our ability to increase coverage and affordability and provide insurance access for patients and choice. The last step is to work toward clinical adoption, supporting the continuing healthcare provider education so that clinicians know what therapies and trials are available, and what people are eligible for these trials in their area. There is great work being done by the clinical trials education team that is put together by Dr. Anastasia Albanese-O’Neil, PhD, that will lead us to better outcomes and the next generation of type one diabetes (T1D) therapies.
JDRF has global universal screening, disease modifying therapies, and cell therapies, and together these three make up the cures research program. Under global universal screening, JDRF has figured out ways to investigate and identify people at risk of developing T1D early before they develop the disease. We know that 85% of people diagnosed with T1D, do not have a familial connection to this disease, meaning that we have to find those at risk beyond just testing relatives of those currently identified as having T1D.
JDRF is focused on rebalancing the immune system and supporting beta cell health and regeneration for disease modifying therapies. Under cell therapies, we look to develop cell therapy products or beta cell sources, implantation techniques, then work to provide or restore insulin independence for those with stage three or who have had T1D for a long time. JDRF also has an Improving Lives portfolio that focuses on devices and technologies for improving glycemic control, psychosocial aspects related to the burden of living with T1D, as well as therapies that address long-term complications. JDRF works to train and bring into the T1D research field the best and the brightest investigators and help them to succeed as the next generation leaders in the field of T1D research. This talk will focus on the DMT portfolio. Simply the DMTs are therapies that include treatments that both control the autoimmunity and preserve or regrow beta cells. JDRF is accelerating the development of these therapies into clinical testing and eventually regulatory approval and access. This has two sides: immunotherapies and beta cell therapies. Under immunotherapies, the question is, how do we turn off the autoimmune attack on beta cells? Under beta cell therapies, the question is, how do we preserve and support functional beta cell mass?
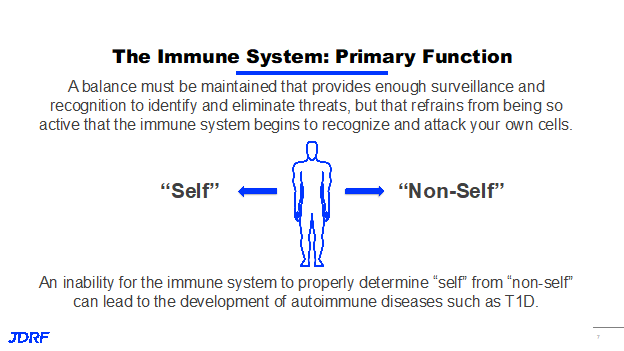
The primary goal of the immune system is simply to tell the difference between yourself and not yourself. It is the body’s ability to recognize when something is a threat, whether it is part of your natural body, or one of your own cells. Your body has to maintain a balance because it needs to provide enough surveillance and enough activity to identify and eliminate any potential threats. You have to refrain from being so active that the immune system becomes too active and starts attacking your body’s own cells. When this overactivity causes the recognition and attacking of your own cells, it is called autoimmunity, and the inability for the immune system to properly determine the difference between self and non-self is the basis where it can lead to the development of autoimmune diseases such as T1D.
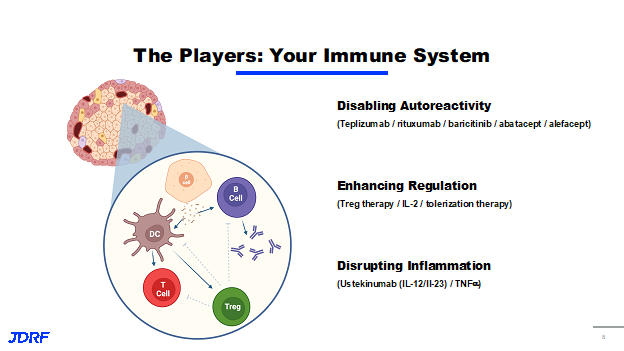
There are a large number of cells and processes involved in the immune response and in autoimmunity. If we look closely within an islet and see a beta cell, the immune system can interact with what is in the immune cells and is able to infiltrate into the islet. They can interact with the beta cell in a number of ways. One of these ways is that they can pick up antigens form the beta cell or little pieces of beta cell. “Antigen” is a word that describes anything your immune system can recognize. This antigen can be recognized by B cells, which activate and then produce antibodies – those are the antibodies against your own body, which are called autoantibodies. These are the autoantibodies that are screened for when looking for people at risk of developing T1D. These antigens can also be picked up by antigen presenting cells, such as dendritic cells, which then show the antigen to the T cells so they will become activated when they see the antigen. They will now attack what they recognize. This attack of the T cells on the beta cell is the basis for autoimmunity and the loss of beta cells and T1D. Finally, there is another T cell which is called a T regulatory (Treg) cell, which is like a T cell, but instead of it attacking it releases chemical signals that cause the rest of the immune system to calm down. It is all about balance. JDRF’s strategy, therefore, is based on this biology of disease. Under the immunotherapy section of the DMT portfolio, we seek to disable autoreactivity. The question is, how do we turn off the T cell attack on beta cells or the B cell response to beta cells? This has been done in a number of trials and approaches, most recently using teplizumab, rituximab, baricitinib, and abatacept, which are all pharmaceutical assets that are focused on disabling this autoreactive recognition by the immune system. Another approach we can take is enhancing regulation. This means supporting the function of these T regulatory cells and allowing them to better control the immune cells around them. This is done in a number of ways by increasing the number of T regulatory cells and has been attempted before through Treg therapy. One of the projects is tolerization therapy and how we specifically activate these cells and tolerize the immune system. Basically, that means the way we promote the recognition of these antigens by the Tregs over the other cells in the immune system. The last concept is to disrupt inflammation. These immune cells activate and cause local inflammation and tissue damage, which then releases more of the antigens for the immune system to recognize coming from the beta cells. We need to work on how we can decrease the amount of inflammation occurring, particularly in the pancreas. There are a number of ways that are being investigated in trials going on with ustekinumab, which acts on IL-12 and IL-23, two of the mediators of inflammation in the body, as well as some strategies looking toward TNF alpha.
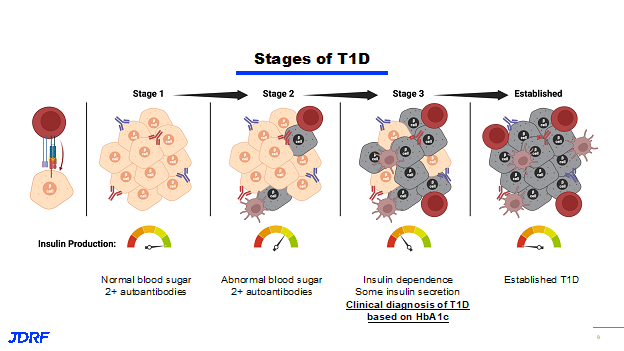
Understanding how the disease develops is important as we talk about the stages of T1D. This more advanced staging graph shows more of what is going on behind the scenes. This will explain what is happening and why we are targeting certain stages of the disease. The most important thing to know about this slide is that recognition of a beta cell by the immune system happens very early on, long before what we call stage one. It happens long before anybody goes and gets tested. The recognition of a beta cell antigen by a cell in the immune system sets off the immune reaction, which can be slow building, but it sets off a growing immune response. People in stage one of the disease have two autoantibodies that are floating through their system which can be detected in their peripheral blood that are specific to beta cell antigens. This means that there is definitely an immune response to the beta cells that is occurring. It has not escalated to the point where you have lost functional beta cells or you have lost insulin production. You are still producing insulin at full capacity. People in stage two, who have two or more auto antibodies are also starting to have some signs of abnormal blood sugars and a slight decrease in insulin production. Stage three is when people are diagnosed based on their HbA1c. This is where insulin dependency starts. You still have some insulin secretion, but you have lost a majority, or at least a good portion, of your functional beta cell mass. You can see that there are more immune cells infiltrating into the islets and more of the functional insulin-producing beta cells as the disease progresses. Finally, in established T1D, you have loss of that functional beta cell component, you have lost the ability of the cells to create insulin, and you are no longer producing insulin at any level.
Last year there was the approval of TZield in stage two. This is a DMT. Tzield works by targeting and altering T cells. T cells, as were shown earlier here, play a major role in the development of disease. They are the cells that are activated and attack the beta cells. TZield was approved November 2022 for people ages 8 and up with stage two T1D. It has been shown that it is able to delay the onset of T1D for nearly three years on average. Recently, the PROTECT trial demonstrated that Tzield preserves beta cell function and can slow the loss of beta cells in children and adolescents with recent onset stage three T1D. This is exciting and means that while it is currently approved for stage two, perhaps now it can move forward for stage three. JDRF is waiting to hear the plans that the company Sanofi will have about moving Tzield forward.
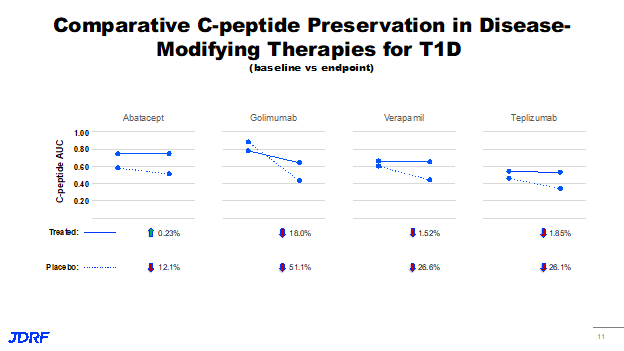
This means that teplizumab joins a number of DMTs that have shown efficacy in stage three or new onset disease. This includes Abatacept, Golimumab, and Verapamil that all showed some preservation of C-peptide, compared to placebo. The effect seen is while the patients receive the drug. It has not yet been determined if there can be long term efficacy. We know that while patients are on the drug, the C-peptide is preserved. There are a number of strategies to do this. This is an important start to see if patients can benefit and from which therapies so we can start to build combinations for providing lasting or more lasting responses and rebalancing of the immune system.
As we know, T1D is a complex disease and the factors that initiate this disease are unknown. This project explores the role of viruses called enteroviruses in the development of T1D. For the first time, there is testing of an antiviral drug in children with new onset T1D. Results from this clinical study showed that those who received the antiviral drug maintained higher levels of insulin production compared to those who did not take the drug. This demonstrates that treatment with antivirals can slow progression of T1D moving the field forward toward an effective treatment. The results are currently published in Nature Medicine.
In this last clinical trial, Swedish investigators will test the use of Diamyd in a phase three clinical trial. Diamyd is an antigen-specific therapy directed toward a GAD autoantigen that works to stop the autoimmune destruction of beta cells. The study is called Diagnode-3 (Flyer) and will include over 300 adolescents and young adults ages 12 – 29 who were diagnosed in the last 6 months. To be eligible, participants must have a certain gene that codes as a T1D risk factor. This is important because this study is the first to use collected samples to determine participants that are likely to respond. There is recruitment at 50 sites in the United States and in Europe.
Another initiative comes to us from Professor Colin Dayan at Cardiff University. There is a critical need to find out what is happening to insulin-producing cells earlier in the disease process so that we can tell whether a treatment is effective or not. This study will test the use of a tool that more precisely measures beta cell function early in T1D using data from the oral glucose tolerance test. If successful, this tool will help us determine within six months whether a drug is slowing the disease process or if another therapy should be used. JDRF is strategically funding new projects to fill gaps and knowledge to bring next generation therapies forward. JDRF is working on therapies that have sustained, rather than had transient rebounds of the immune system. A way that we can do this is by testing combination therapies.
Out of the many types of immune cells that are involved in T1D, B cells and Tregs play a crucial role in the process. While B cells enhance the autoimmune process, Tregs try to restrain it. Dr. Wasif Kahn, PhD, will test the use of two drugs in combination. One of the agents will promote Tregs while the other drug BTK. A BTK inhibitor will dampen B cell maturation and cause B cell depletion. This project will test the efficacy of combining therapies that enhance Tregs while getting B cells to stop or significantly delay T1D progressions. We do not fully understand why T cells and autoimmunity do not become exhausted like they do in other instances, such as cancer or prolonged infection. Dr. Thomas Kay has developed a team of 11 experts in fields ranging from T1D biology to drug development. It is exploring the question and developing a therapeutic agent for the use of T cell exhaustion to shut down T cells driving disease and T1D. This project was developed under the cures collaborative RFA with a goal of using combined expertise and resources to drive development from discovery research all the way to a therapeutic agent to validate testing.
Another project comes from an investigator who is new to T1D. Dr. Andrea Sheitinger is a cancer immunologist who is now applying her knowledge from cancer biology to T1D to determine how autoreactive T cells are maintained in T1D. Dr. Sheitinger previously identified a subset of autoreactive T cells that express a protein that maintained stemness and self-renewal called TCF-1. It was a surprise that as few as 10 of these T cells can initiate disease in mice. This project will identify critical elements contributing to how TCF-1 positive T cells arise and how they are sustained with the goal of reprogramming the cells to prevent beta cell destruction.
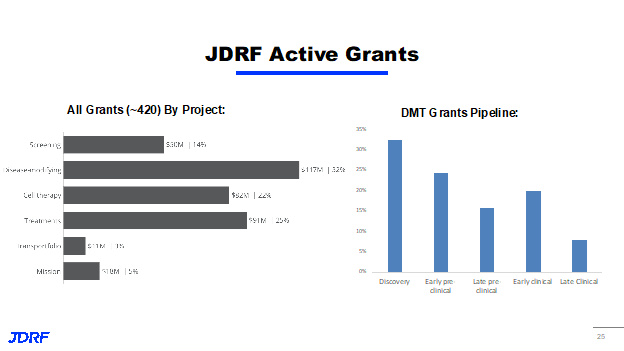
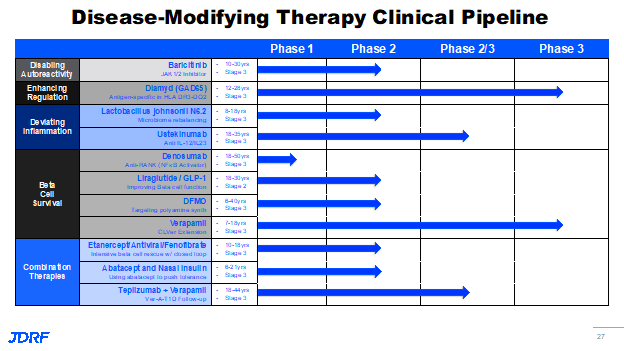
There are currently 420 active grants in the JDRF research portfolio, roughly 32% (or $117 million worth of funding) fall into DMT, which are cure therapies. This means it is a substantial portion of the overall research portfolio. These grants are under the DMT portfolio and fall across all stages of research from discovery to late clinical. The numbers represent the number of projects under each priority area of DMTs, as well as stages of development. JDRF is funding projects in each of the priority areas from disabling auto activity, enhancing regulation and deviating from inflammatory processes. There are currently 11 clinical trials funded under the DMT portfolio, in early clinical and late clinical trial development. In these clinical trials span the breath of the work being done by JDRF. This talk discussed Baricitinib and Diamyd as two ongoing trials that we will be excited to see the reports from.
Baricitinib is currently a phase two BANDIT trial. Diamyd, Diagnode-3, is a phase three trial. JDRF currently has three combination approaches that are in clinical development. There are other intensive beta cell rescue combination trials also in progress.
Three of the trials we are highlighting are currently being run by TrialNet that is funded by the Special Diabetes Program. JDRF is instrumental in getting the support for this from Congress. JDRF advocates are an important reason why this T1D funding of over $150 million a year has been passed. Members of Congress need to hear from you to get this funding renewed. One is called STOP-T1D, which is the trial of anti-thymocyte globulin ATG Prevention study. It is a study of high-risk individuals between the ages of 12 and 35. This trial is testing low dose ATG, which acts on the T cells. Its activity is in disabling auto-activity.
The JAKPOT-T1D trial is looking at a combination of two JAK inhibitors, abrocitinib and ritlecitinib, in newly diagnosed individuals ages 12 to 35 years of age.
The last one is T1D RELAY which combines two immunotherapies, rituximab and Abatacept, in newly diagnosed individuals ages 8 to 45 years old.
The goal is to work on therapies that include treatments that control autoimmunity and preserve or regrow beta cells. JDRF is accelerating the development of these therapies into clinical testing, and eventually regulatory approval and access. There are two questions that are asked in the DMT portfolio. For immunotherapies it is, how can we turn off the immune attack on the beta cells? Under beta cell therapies the question is, how do we preserve and support functional eta cell mass? If we zoom in on the beta cell therapy section of the portfolio, there are three aims that can be focused on. One is to preserve beta cell survival and function. Another is to increase functional beta cell mass, and the final one is to create new beta-like cells form other cell types. This is what will be covered in the last part of this article.
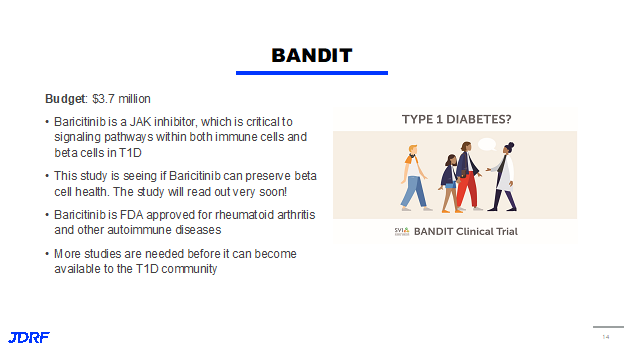
There has been exciting news that has just come out on the BANDIT clinical trial with baricitinib, which is a JAK inhibitor. JAK inhibitors are critical to the signaling pathways that are involved in immune cells and with beta cells. It is currently approved for treating a range of diseases like rheumatoid arthritis, hair loss or alopecia, and COVID-19. It is important to note that this is a daily oral pill. This JDRF-funded clinical trial, which was just published in the New England Journal of Medicine, showed in 60 newly diagnosed children and young adults that baricitinib is able to preserve beta cell function as measured by C-peptide levels. It also improved blood sugar variability in time in range and decreased the requirement for external insulin. Along with these positive results, it also showed a very favorable safety profile.
Part of this article was from the talk done by Drs. Jay Tinklepaugh and Joshua Vieth.
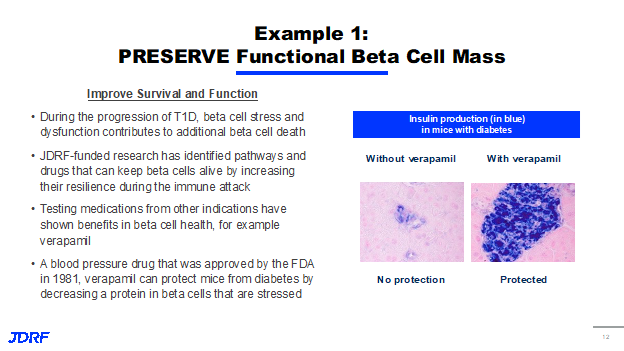
In preserving functional beta cell mass, one of the first lines of intervention that we can employ in T1D is simply to improve the survival and function of beta cells in response to the stress and attacking of the immune system, effectively identifying ways to preserve beta cells. As a reminder, we know that during the progressions of T1D, beta cell stress and dysfunction contributes to additional beta cell death – it is not just the damage done by the immune system. JDRF-funded research has identified pathways and drugs that can keep beta cells alive by increasing their resilience during the immune attack. There has been quite a bit of success lately, and historically, in testing medications from other indications that have shown benefits on beta cell health. A notable example of this is Verapamil, a blood pressure drug that was approved by the FDA in the early eighties.
You can see the figure on the right, Verapamil treatment can protect mice form diabetes by decreasing a specific protein in beta cells when they encounter stress. It shows that Verapamil has a protective effect and maintains insulin production in a mouse model. This was driven by JDRF support and funding of clinical trials that was nuanced to T1D adults and children, which helped show that Verapamil aims to slow progression by helping maintain C-peptide. You can see on the right that those taking Verapamil will need less insulin and have better overall glycemic control than those on a placebo treatment.
There is now a clinical trial of Verapamil alone and with immunotherapy treatments that is being carried out by the European consortium INNODIA, which is a public private project co-funded by JDRF. In addition to repurposing Verapamil and moving that through the clinical pipeline, there is also on ongoing trial with Drs. Emily Sims, MD, and Linda Dimeglio, PhD, focused on targeting T1D through polyamines with DFMO. In the past JDRF ran a clinical trial to see if a sleeping sickness drug works to improve the health and function of beta cells. Study participants undergoing treatment actually had healthier beta cells with better insulin production compared to those taking the placebo. This project aims to test this therapy in a larger number of people for six months, relative to three done in the earlier study to see if the earlier results will hold up and we are able to help sustain healthy beta cell populations. If successful, the clinical trial will confirm this novel approach to beta cell preservation and form the basis for additional trials to help delay T1D onset in at-risk individuals. JDRF is also supporting a phase one clinical trial with Dr. Rupangi Vasavada, PhD, focused on examining the impact of an osteoporosis drug called denosumab on beta cell health. Historically, we have known that beta cells are injured by a pathway that is also known to cause bone loss. Dr. Vasavada has proposed that we use an osteoporosis drug to help block this pathway and improve the health of human beta cells and protect them against injuries that are relevant to T1D, effectively delaying or reversing the disease. This will be a phase one clinical trial; the drug will be given four times a year and we will observe whether or not it improves beta cell function and glucose balance in people who have early onset T1D.
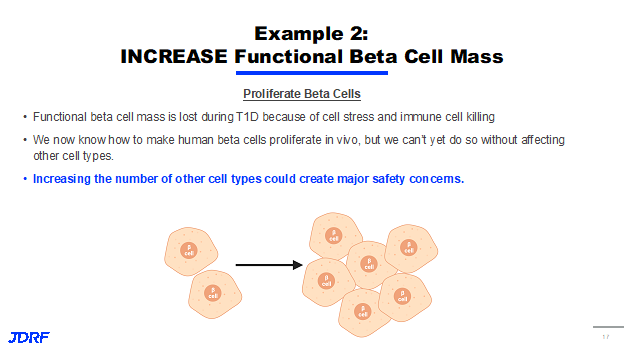
Another approach is to increase beta cell mass, and is focused on finding ways to increase the number of beta cells that have survived the immune system attack and corresponding beta cell stress. The goal would be to replace the beta cells that were lost by encouraging the survivors to multiply and restore their overall number as shown in the figure above.
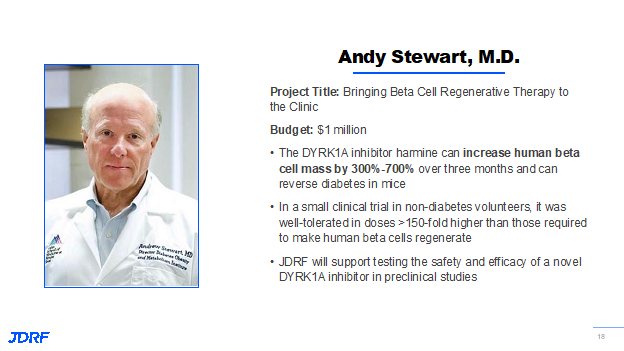
For many years, beta cells were considered part of a small subset of cells within the body that are non-proliferative, which means that they do not multiply and reproduce by themselves. Thanks to JDRF-funded research, there have been several exciting discoveries in this area over the years. One notable example was the JDRF partnership with GNF, Novartis that provided some of the original exciting data suggesting that we can increase the beta cell population in the body. However, there are concerns that these strategies are not beta cell-specific, and that they might impact other parts of the body. This is a huge problem because increasing the number of cells in the wrong parts of the body could create safety concerns. JDRF is supporting projects in these areas that include both targeted and non-targeted approaches to the beta cell. One very exciting project in this area is from Andy Stewart, MD, who proposes the development of a new DYRK1A inhibitor in preclinical studies to help increase functional beta cell mass. Historically, JDRF research has identified DYRK1A as a promising target to increase beta cell mass in mouse models. An inhibitor, harmine, increased in both human and mouse models by 300 to 700% over three months. In addition, in a small clinical trial in nondiabetic volunteer, harmine was given in doses over 150 times higher than those required to make human beta cells regenerate, and there were no significant safety concerns. Another approach involves creating new beta cell mass or new functional beta cell mass from other tissues or other cell types within the body. We have known for a long time that as T1D and type 2 progress, other cells in the pancreas try and change into beta-type cells. Unfortunately, not enough of them are able to make this transition to restore the insulin supply to the body. If we approach this either through gene therapy, or non-gene methods, there may be a way in which we can deliver signals to other cell types in the body to force them to be more like beta cells to produce insulin and make up that deficit in T1D.
JDRF is researching ways to use either the gene therapy or chemical interventions to deliver these signals and develop ways to control this approach, but deliver them effectively and in a safe manner to ensure that only the cell types we want to change are impacted. There is an exciting project in this area that is being supported by JDRF in the lab of Dr. Stefan Kubicek, PhD, who is investigating how pancreatic alpha cells can become beta-like cells through small molecules that induce insulin expression. With JDRF support, Dr. Kubicek, in a genetic screen found four proteins that without these results in increased insulin and alpha cells. With this knowledge, he is developing small molecules that can effectively downregulate those proteins and hopefully restore insulin production. To summarize, there are a number of exciting projects focused on preserving, increasing, and creating new beta cell mass in T1D. We hope to advance these strategies alongside or in combination with promising immune-focused therapies. There are still major barriers to progress in this area. JDRF is addressing these barriers.
For more information on these trials, you can go to www.jdrf.org/clinicaltrials . One of the new sections on that website is a featured trial section, which highlight a new trial every month that is recruiting to keep everyone up to date on the latest information.
If you have any questions on this article or video, please contact me at debbieaevans1@gmail.com. I am also one of the CTEV (clinical trial educational volunteers) for the JDRF Chapter Minnesota and Dakotas and can answer many questions on clinical trials including for those newly diagnosed. These trials often have a short enrollment period and a participant needs to be enrolled shortly after learning that they are T1D. The trials are often look to see if a treatment will help the beta cells in some way early in the process.