DISEASE MODIFYING THERAPIES PART TWO
Dr. Joshia Vieth, PHD JDRF Director of Research
Dr. Jay Tinklepaugh, PHD JDRF Scientists
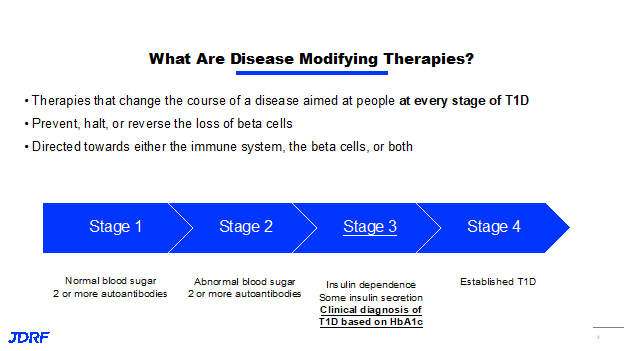
Dr. Joshua Vieth, Director of Research for JDRF, presented information recently discussing what JDRF is currently studying in disease modifying therapies. There is much to discuss as there are many research therapies moving through the disease modifying therapies pipeline. Disease modifying therapies are therapies that can change the course of type 1 diabetes (T1D). These therapies are targeted at every stage of T1D, from stage one where you have normal blood sugar and you are expressing autoantibodies, all the way to stage four where you have established T1D. The goal of disease modifying therapies is to prevent, halt or reverse the loss of beta cells. The therapy can be directed either towards the immune system, the beta cells, or both. JDRF is working on two strategies with one being to turn off the autoimmune attack against the insulin producing cells by the immune system, and the second is creating and sustaining beta cells in the body. This second part will be covered later in this article. Both goals include treatments that both control autoimmunity and preserve or regrow beta cells. JDRF is accelerating the development of these therapies into clinical testing, and eventually into regulatory approval and access to clinician and patients.
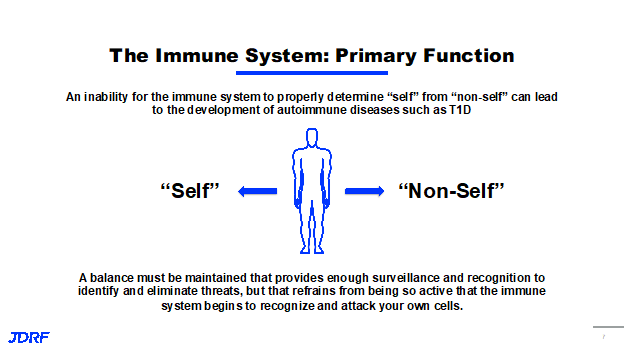
One of the main goals of the immune system is to monitor what is self and non-self. An inability for the immune system to properly determine self or non-self can lead to the development of autoimmune diseases such as T1D. New therapeutic options that are being developed will need to create a balance with and maintain the ability of the immune system to work at surveillance, recognition and elimination of threats. It is also important that it is not so active, that the immune system recognizes and attacks its own cells. If we think of the stages of T1D, the important thing to remember is the immune events that turns the immune system against itself, happens long before stage one. This is why screening for autoantibodies is so important. Those autoantibodies can be screened through TrialNet and with T1Detect. It is important to study what leads up to the events that cause immune activation and have new therapies that can not only stop this attack, but can also treat patients to reverse it at all stages.
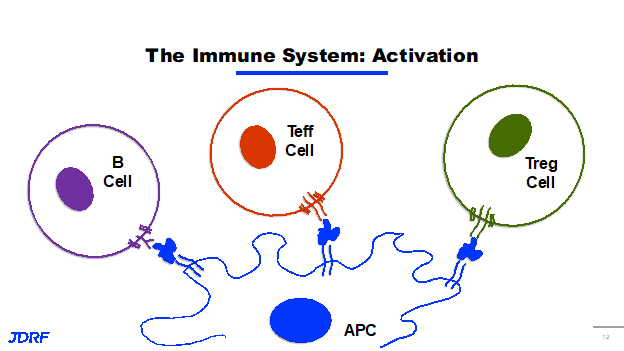
JDRF discovery research is involved in identifying new targets and testing new ideas for therapeutics for T1D. This development research that ultimately feeds the pipeline, ensures that JDRF continues to support studies and science that represent the next round of clinical trials and potential cures for T1D. JDRF’s strategy for turning off the immune attack against beta cells is threefold. First, we want to turn on the brakes of the immune system and slow it down. Second, we want to interrupt the recognition of beta cells by the immune system. Finally, we want to turn around and turn down the inflammation and the negative effects that come along with immune activation. When talking about immune activation, it is really about the process of recognition by the immune system and its antigen recognition. An antigen is really any particle that is recognized by and can activate the immune system. We generally think of these as potentially bacteria or viruses that invade the body and need to be eliminated. It could also be a toxin or protein that needs to be eliminated. In autoimmunity, the body mistakenly thinks its own antigens are an invader. An antigen in its simplest form is anything that can be recognized by an activated immune system. The cells in our immune system are able to take in these antigens, and then present them on their surface to other cells in the immune system. These cells are called antigen presenting cells (APCs) and can be dendritic cells and macrophages. Antigen presenting cells flag an antigen and present it to an immune cell, another immune cell recognizes it and then will go to do a certain job. When this antigen is from a cell in your own body, it is called an autoantigen. Autoantigens are antigens from you own body that are being recognized by an active immune system. In T1D, your immune system is recognizing autoantigens from beta cells. The immune response or activation is what makes T1D an autoimmune disease. This is the basis of the JDRF research strategy in disease modifying therapies.
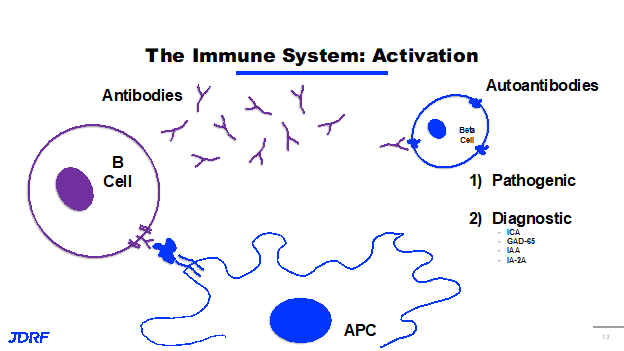
The immune system is very complex. There are three cell types in our immune system that recognize antigens presented by antigen presenting cells. These are B cells, types of T cells including T effector cells (Teffs), and T regulatory cells (Tregs). When a B cell recognizes an antigen that’s being presented, it activates and produces antibodies. These antibodies are small Y shaped proteins that also identify, target and recognize antigens. These cells go out and tag these antigens for destruction by the immune system. It is like displaying a flag saying it is time to get rid of it. In autoimmunity, it is your own cell or antigen on your beta cell that is a protein or antibody and is now called an autoantibody. These autoantibodies can be pathogenic, meaning that they can mark your cells for destruction and lead to further immune activation and further progression of T1D. Some of these autoantibodies are considered diagnostic and they do not necessarily activate the immune system further. The diagnostic part is that they are an indicator that your immune system has already started to recognize your beta cells and there is a mounting immune response against them. Some of the common diagnostic autoantibodies in T1D are ICA, GAD65, IA-2, IAA’s and IA-2A. These are the autoantibodies screened for in stage one and stage two of T1D.
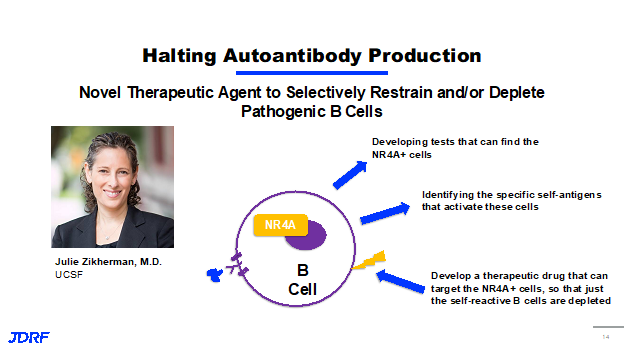
JDRF is funding research to halt this autoantibody production and the activation of B cells. B cells produce these antibodies. Some of this work comes from Dr. Julie Zikherman, MD at UC-San Francisco. She is investigating a new way to identify B cells that recognize self-antigens from beta cells. Her focus is on a family of proteins called NR4A that is expressed in B cells that are receiving a constant ghost signal or a constant activation signal. This suggests that these are B cells that are ultimately responding to the self-antigens, perhaps the antigens from your beta cells. Dr. Zikherman is developing tests that can find these NR4A positive B cells to identify them in patients. She is also using this ability to identify these cells to figure out which specific self-antigen is activating these cells. This information can be used to develop a therapeutic drug that can target only these NR4A positive cells. The therapy idea being that the cells that were these active B cells that are recognizing your own antigens can be depleted and will leave the other B cells in the immune system that can do their job normally and continue to work.
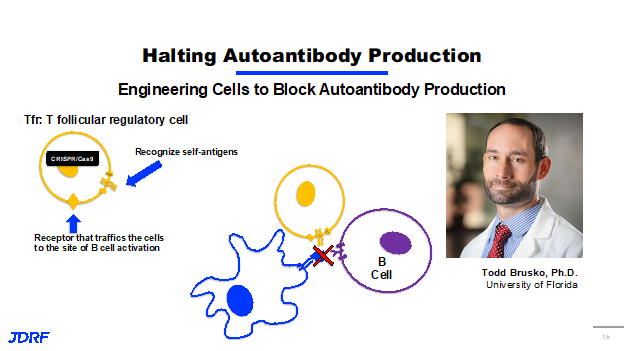
Another researcher, Dr. Todd Brusko, PHD at the University of Florida, is also working in the field with B cells. JDRF has a history of funding Dr. Brusko through a postdoctoral fellowship, career development award and now through this ongoing research agreement. This also highlights the importance of investing in young scientists and bringing them into T1D research. It allows JDRF to develop the next leaders in the field. Dr. Brusko’s idea is that B cells that produce autoantibodies are an early target for halting or reversing the onset of type 1 diabetes. He also works with another type of cell in the immune system called a T follicular regulatory cell. The T follicular regulatory cell is able to stop B cells from being activated. Cutting edge gene engineering such as CRISPR cas 9 technology is used to create T follicular regulatory cells that not only have another receptor that can recognize the beta cell self-antigens, it also has another receptor that traffics or causes the cells to migrate to areas of the body where the B cells are being activated. The idea here is that when you have the presentation of a self-antigen to a B cell, these cells will be present to inhibit that activation and stop the B cells from being activated and producing autoantibodies. Dr. Brusko is currently doing test to see if these engineered cells can halt T1D in an animal model in preparation for further clinical testing.
The next type of cell to focus on is the T effector cell. These are the cells that once activated will go out to find and destroy their target antigens when recognized. When a T effector cell recognizes antigens presented by the antigen presenting cell, it will activate and proliferate. This means it will make multiple copies of itself. Each of these copies will recognize the same antigen and go out to try find this antigen and destroy the cells associated with it. In the case of T1D, this antigen happens to be on a beta cell and when one of these T effector cells recognizes it, they activate and then produce a chemical called a cytokine. Cytokines are small secreted proteins released by cells that have a specific effect on the interactions and communications between cells. These cytokines have a number of results. First, they cause inflammation in the surrounding tissue. This is why sometimes pancreatitis or tissue inflammation is associated with T1D. The cytokines can actually destroy the cell that is recognized and this destruction of the beta cell will cause the release of more antigens that will then cause further activation of the immune system. This is a process that ramps itself up.
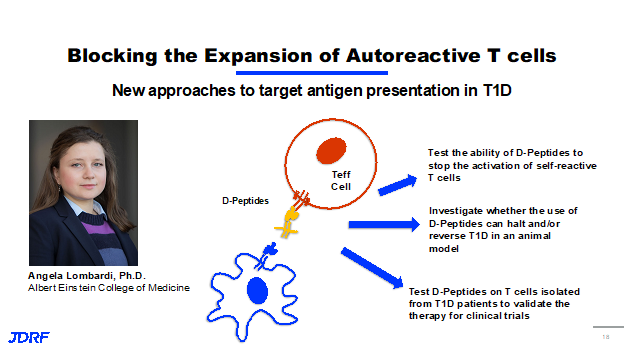
Other work that JDRF is funding in this area is from Dr. Angela Lombardi, PHD at Albert Einstein College of Medicine. She is looking to block the expansion of these autoreactive T effector cells. Dr. Lombardi has developed a small molecule called a D-peptide. These are basically synthetic versions of the antigen that is recognized by your own T cells, connected to a protein that mimics the protein the presentation from antigen from the antigen presenting cells themself.
These are basically synthetic versions of the antigen that is what self is recognizing and reacting to. The synthetic version is attached to a cell that can mimic the antigen presentation.
This modifies it in a way that when this small molecule binds to your effector T cells, it doesn’t allow them to be activated. Because these D-peptides are bound to your T effector cells, it also stops the antigen presenting cells from presenting the antigens from your beta cells, meaning that the goal here is that the T effector cells are never activated. They are unable to go out and attack your beta cells further. Currently, Dr. Lombardi is testing the ability of these D-peptides to stop the activation of the self-reactive T cells. She’s investigating whether you can use the D-peptides to halt and reverse T1D diabetes in animal models. She is also doing work in T cells isolated from T1D patients to validate this as a therapy for clinical trials. Dr. Lombardi was funded during a one year JDRF innovative award. It is nearing completion and has been very successful. This work is being expanded into a fully funded NIH study. She is looking to initiate a phase one clinical trial for the use of D-peptides. This is a great example of how important the innovative grant program is at JDRF. It really enhances our ability to facilitate the transition of great novel ideas and discovery research into the next clinical trials and therapies.
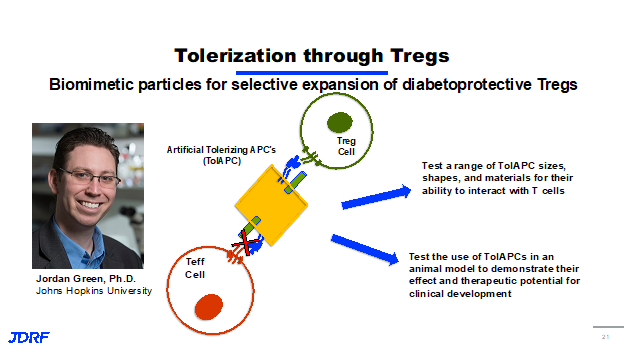
The last cell to discuss is the T regulatory cells, or T regs. The best way to think about the T regs is that these are the good guys of the immune system. These are the cells that once activated actually go out and then slow down the immune process. They shut down the immune system so it does not become too active. Like other T cells, they recognize antigens that are presented, they activate and proliferate by creating more copies of themselves that recognize the same antigen. What differentiates them from other T cells, such as T effector cells, is that when a T reg recognizes an antigen on a beta cell, instead of producing those inflammatory cytokines, the chemical that cause destruction, they produce chemical messengers, that actually calm down and soothe the inflammation. If there are T effector cells in the area that are attacking beta cells, these chemicals turn off the immune response against the beta cells. For this reason, T regs are a really important focus of therapy for our disease modifying approach. This is a process called immune regulation. This is the goal, we want to be able to highlight the body’s ability to regulate its own immune system, and have a targeted way to shut down the self-reactive immune response. JDRF is currently funding Dr. Jordan Green, PHD at Johns Hopkins and Dr. Green is a biomedical engineer. He has a very unique approach to try to tolerize or turn off the immune system. Using T reg cells, Dr. Green has developed what he calls artificial tolerized APCs, antigen presenting cells. Dr. Green is creating a synthetic version of these out of a number of materials. He is calling these VCs or artificial tolerating polarizing APCs. These synthetic molecules have the beta cell antigen that’s recognized by T cells on their surface, as well as another molecule that can draw only activated T regulatory cells. This molecule inhibits the activation of T effector cells. In effect, when you add these tolerating APCs, they flip the switch for the immune system into more of a protective state instead of an effector or an attacking state. Dr. Green’s work includes testing a range of these synthetic molecules for size, shape and also the materials they are made of to see how they interact with T cells. He is also testing their use in animal models to demonstrate their effectiveness and whether they could have therapeutic potential for clinical development.
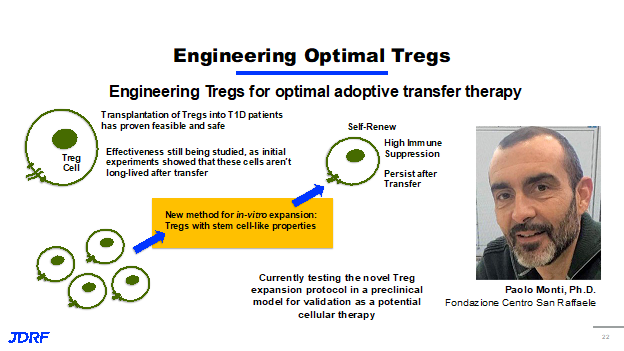
Finally, JDRF funds research around the world and a great example is the work being done by Dr. Paolo Monti, PHD, at San Raffaele Scientific Institute in Milan, Italy. JDRF funded Dr. Monti through his postdoctoral fellowships, through career development awards, and all of which were very successful and resulted in the ongoing work in his lab that JDRF is also funding. He is focused on identify and developing T regs that are optimal for immune suppression. Past work in Dr. Monti’s lab has shown that transplantation or putting T regs into T1D patients is both feasible and safe. You can place these cells into patients and they do shut down the immune response. However, initial experiments haven’t been overly successful because it shows that these cells while they initially have an effect on the immune system, they aren’t long lived after transfer. So they do not last very long in a recipient. More work is needed to make this an effective approach to having lasting immune tolerance. Dr. Monti’s ongoing work has taken T cells from those with T1D. He has developed a new method in this laboratory for expanding and activating these cells in vitro, meaning outside of the body or basically in a test tube or lab plate. This new method that Dr. Monti has developed, gives these T regs, stem cell like properties. When he takes these T regs and puts them back into his model system, he shows that they can self-renew. They continually make copies of themselves, so they stay around. They also have a very high immune suppression potential, meaning that they work even better than normal T regs. They persist after transfer, meaning that this is potentially getting past our issue with T regs not being long lived. This could be a lasting therapy for those with T1D. Dr. Monti is currently testing this novel protocol, in a preclinical model and validating it as a potential cellular therapy in clinical trials. The hope is that this discovery research that JDRF is funding leads to the future therapies, the future clinical trials. The takeaway message is that JDRF is funding discovery research in the US and around the world, Work is being done to develop a wide variety of approaches in multiple parts of the immune system to prevent or stop the immune attack on beta cells, and eventually develop a cure for type one diabetes.
Jay Tinklepaugh works at JDRF as a scientist. He recently discussed the targeted therapies that JDRF is working on for creating and sustaining beta cells. This is part of the ongoing work to develop and advance disease modifying therapies. JDRF is supporting projects that are centered on finding new proliferative targets to encourage beta cells to replicate or proliferate. JDRF is also supporting projects that are implementing new targeted drug delivery based approaches. The goal is that promising drugs that help the beta cell either proliferate or regenerate are delivered only to the beta cell and not to other cells throughout the body. To address this challenge of how to create and sustain beta cells, JDRF has really broken this down into three broad areas, identifying new ways to improve beta cell survival and function, finding ways to improve the function of beta cells that have survived the immune attack and encourage them to replicate or proliferate and finally finding ways to convert other cells into insulin producing beta like cells.
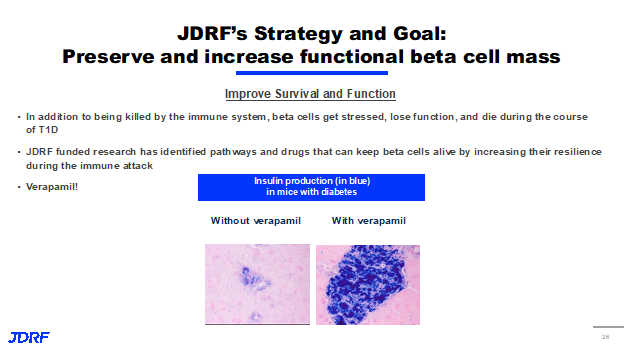
The focus for JDRF, is in trying to protect the beta cells and encourage more beta cell growth. In thinking of the four distinct stages of T1D, currently most disease modifying therapies are targeted at the stage three population, that’s due to a number of reasons. One of them being that many individuals in stage three still have a measurable number of beta cells remaining. Thus, there’s a reasonable number of targets to deliver a drug to. These projects are starting in the stage three population, the goal is these beta cell focus strategies will help those in all stages of T1D, including those with established T1D in stage four, who may only have a very small number of beta cells left and those in stages one and two, keep them from progressing to insulin dependence. The first approach is centered on finding new ways to improve the survival and function of beta cells in response to stress and the attacking immune system, not every beta cell dies solely due to the immune system, and when the beta cells are stressed, they can begin to lose function and ultimately die during this time. Previous JDRF research has identified several different pathways and drugs that can keep beta cells alive and functional by making them more resilient to the immune attack. An example of a drug that we think does this is Verapamil. Verapamil is a drug primarily used to treat high blood pressure. But in mice with diabetes, it has been shown to reduce beta cell death, which you can see in the figure below. Diabetic mice that were not given. Verapamil had much more beta cell death and a significantly less insulin production than those that received the drug. Currently, JDRF is supporting Verapamil investigation in clinical trials to see if that same protective impact is seen in humans.
The second approach is focused on finding ways to get the beta cells that have survived the immune system attack and associated stress to proliferate or multiply. The goal of this would be to replace the beta cells that were lost by encouraging the survivors to multiply and restore their overall number.
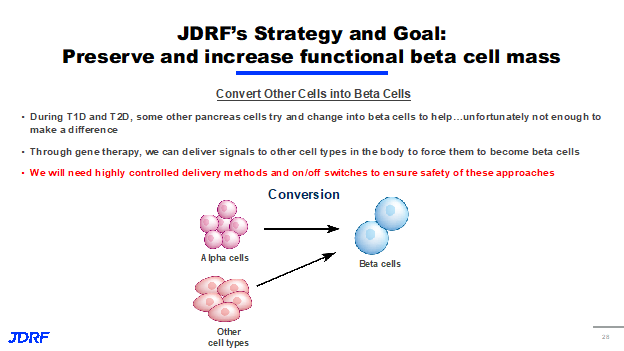
There have been several exciting discoveries in this area and we now know how to make beta cells proliferate in vivo, but work is still being done to achieve tissue specificity. This is a problem that JDRF is trying to overcome because proliferating cells in the wrong parts of the body could create some major safety concerns. This is a major objective of the targeted drug delivery projects that will be discussed here also. The third approach we are taking involves the conversion of one type of cell into something that much more closely resembles a beta cell. As you may or may not know as T1D and type two diabetes progress (T2D), other cells in the pancreas try and change into the beta cell to help out and supply insulin to the body. Unfortunately, not enough cells are able to change from one type to another to make a difference in insulin being made. T1D’s still need insulin to manage type one. We are studying ways to use gene therapy to deliver signals to other cells both inside the pancreas and out to force them to become beta cells. Similar to the proliferative approach, we will need to be very careful and we will need highly controlled delivery methods and on/off switches to ensure that these strategies are implemented safely.
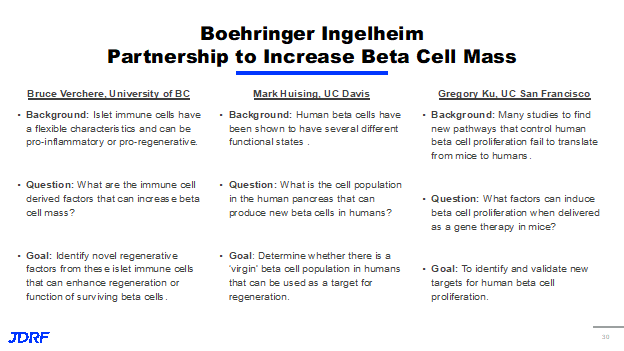
While there are definitely some very exciting tactics in progress in this area, there are still significant hurdles remaining. Mainly, can we induce proliferation and beta cells and or reprogram other cells safely without having dangerous off target effects. In order to address these problems, we’re coming at them from a number of different ways. First, we’re continuing to work on identifying new proliferative targets and strategies and there is ongoing work in that area through a partnership between JDRF and Boehringer Ingelheim. Second, in order to minimize the risks associated with inducing beta cell proliferation and cell reprogramming, we are pursuing a number of projects and targeted drug delivery. Through a partnership between Boehringer Ingelheim and JDRF, there are three research projects really focused on finding new ways to proliferate and regenerate the beta cell. To reiterate, in the meaning to have cells proliferate, we want the existing beta cells to multiply or replicate. We want one to turn into two and then four and so on. Whereas with regeneration, we want a stressed and potentially unhealthy beta cell to return to a healthy functional state. The first project is with Dr. Bruce Verchere at the University of British Columbia and it’s based on the observation that certain immune cells can have flexible characteristics, with some being very proinflammatory and less harmful to beta cells and others being more beneficial and proregenerative activists. Dr. Verchere is working to identify the characteristics of these immune cells and potentially what they secrete that can help increase beta cell mass and hopefully find ways that these factors can enhance survival and the function of surviving beta cells.
The second project is with Dr. Mark O. Huising, PHD, at University of California- Davis, and is built on the observation that human beta cells have several different functional states. Some beta cells are mature and are producing insulin and others are at different stages in their development. They haven’t quite reached that point of producing insulin. Dr. Huising’s project is really focused on identifying these different cell populations in the human pancreas, and determining which of them can produce new beta cells and which of these populations might be the best for potential regenerative therapies. The hope is that these virgin beta cells, as he calls them, will be a promising target for the targeted drug delivery approaches that JDRF is also pursuing. The third project is with Dr. Gregory Ku at University of California-San Francisco. This is based on the well documented observation that in many studies to identify new pathways controlling human beta cell proliferation fail to translate from mouse models into humans. Dr. Ku is taking a different approach to this problem and is attempting to use gene therapy to find new factors that trigger beta cell proliferation. The ultimate goal of this being to find new ways to both identify and validate new targets for human beta cell proliferation.
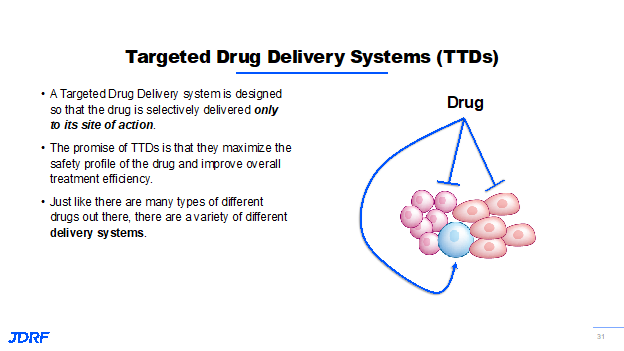
In addition to finding new ways to help beta cells replicate and be healthier overall, we’re also working to develop ways to safely deliver drugs to the beta cell. This is being done via an exploration and implementation of targeted drug delivery systems. Targeted systems are designed that any drug is delivered only to its site of action. For example, you wouldn’t want a plane that you expect to drop you off in the sunny tropics to let you out in the Arctic Circle. Well, the same goes for drug treatment. As shown below, we want to ensure that the drug is only going to the beta cell, the blue cell in this example, and not to any of the others throughout the body.
The promise of this approach is that it mitigates the safety risk, everything becomes safer, and it decreases the amount of drug that we would actually need for any treatment because it’s just a more efficient approach. So just like there are any number of ways your eye could get from A to B, there are a number of different drug delivery systems being looked at by JDRF. This includes using beta cell specific proteins as a form of drug shuttle with nanoparticles loaded up with regenerative drugs or compounds targeted specifically and only to the islets, and even viruses that will selectively reprogram certain cell populations and only those populations into beta like cells. In this area of research, the first project to be discussed here is a partnership between Dr. Maike Sander at University of California-San Diego and Ionis pharmaceuticals. Ionis has developed a peptide based technology that is able to deliver therapies directly to a human beta cell. Alongside, Dr. Sander developed with JDRF support a compound called an antisense oligonucleotide that can help make human beta cells proliferate in vivo. JDRF has helped to create a partnership between Ionis and Dr. Sander to deliver these proliferative agents to the beta cell and they are currently being tested by Dr. Sander in mouse models of T1D. If this project is successful, it would be the first company to have a targeted drug system that can make beta cells proliferate, and this would be a very promising step on the path to a cure.
The second project is with Dr. Paolo Serafini at the University of Miami, and Paolo’s research is focused on using nucleic acid aptamers made of either DNA or RNA to deliver drugs specifically to the beta cells. These aptamers , the short sequences of DNA or RNA, are very special due to the fact that they can fold into a shape that will fit only a very small specific target, think of a lock and key. This folding pattern allows the aptamer to bind tightly to its target. We can deliver a drug with very high specificity. So what Paolo’s lab is currently working on are two of these platforms, both targeted to the beta cell but designed to deliver different therapies. One is designed to improve beta cell function and potentially encourage proliferation where the other one is designed to help alleviate the immune attack on the surviving beta cells. His lab is currently testing these to determine how effectively they delivered the target and modifying the cell.
The final topic is a company from the T1D fund called Code Bio. Code bio is using scaffolds made from slightly modified DNA to serve as a customizable drug delivery platform. There is a DNA scaffold framework, the double stranded helix can then be customized with different antibodies, different drugs, different imaging agents, thus many possibilities. It is really this flexible nature of the platform that makes the Code Bio scaffold so exciting because in addition to all of the different therapies that can be attached, this can be modified with almost any type of targeting agent that confers specificity to the beta cell. This could be a peptide and antibodies, small molecule, numerous different options. In summary, current targeted drug delivery approaches are predominantly in the proof of concept stage in T1D, but at JDRF and across the field all are working to keep them moving forward. Right now, Some of these projects include nanoparticle delivery platforms, additional viral gene delivery methods, antibody drug, conjugates, and so on. We haven’t ignored the immune system in this targeted drug delivery space as well. We are looking at how these targeted delivery strategies can be applied to the immune system and how the lessons being learned around targeting beta cells can also be applied to immune cells as well. And looking forward. The future of disease modifying therapies is very promising. JDRF will continue to identify discovery research that focuses on new and expanding areas of immune intervention work to develop new combination therapies for type one diabetes that addresses both immune dysfunction and beta cell function. In addition, to continue to find and expand the number of targeted drug delivery approaches we have for both the immune system and the beta cell and really continue to drive discovery research into the clinical setting with the goal of developing a cure for T1D.