What Drug Will Be the Next Tzield?

Last year, Tzield became the first disease-modifying therapy (DMT) approved for type 1 diabetes (T1D). JDRF’s disease-modifying therapy pipeline is stocked with other therapies that have the potential to join it as an approved therapy in the US and worldwide.
Disease-modifying therapies are one of, if not the, top success stories of T1D research in 2023. From CLVer to BANDIT to PROTECT, there have been multiple updates on various therapies that can help people with T1D and those at risk for developing it.
This blog highlights eight disease-modifying therapies, including one that is FDA-approved for those at risk for developing T1D, their effect in T1D, current research, and more.
Let’s dive in!
What Are Disease-Modifying Therapies?
Disease-modifying therapies are one of the pillars of JDRF’s strategy to accelerate the development of cures for T1D.
A disease-modifying therapy is exactly what it sounds like—a therapy that alters the course of a disease. A DMT for T1D is any therapy that slows down the progression of T1D, halts its progression, or reverses it entirely.
JDRF is focused on two main tactics to develop DMTs for T1D:
- Stop the immune system from attacking healthy beta cells, preventing T1D from ever occurring.
- Spur beta cell growth safely and protect beta cells, so people with T1D can live without life-long external insulin.
If we can discover a therapy, or combination of therapies that prevent T1D onset and, eventually, reverse it altogether, we will have discovered cures for T1D.
“Type 1 diabetes does not happen all at once—it’s a progression,” said Esther Latres, Ph.D., JDRF vice president of research. “For some, the autoimmune process has begun but they are asymptomatic. Others have had T1D for decades and make virtually no insulin. JDRF is focused on DMTs that can help everyone with T1D, no matter where they are on the disease continuum.”
The Path Toward Disease-Modifying Therapies
Thanks to decades of JDRF-funded work, multiple potential disease-modifying therapies have shown efficacy in altering the course of T1D in people—not just in mice. One of them, Tzield, received FDA approval and is being used in individuals at risk for developing T1D.
There’s also, thanks to JDRF and our collaborators, a regulatory pathway for DMTs to receive FDA approval.
Right now, there are multiple disease-modifying therapies approved for other diseases that have shown they can protect and preserve beta cell health in people with T1D.
People who receive these therapies after diagnosis continue to make more of their own insulin, measured by the amount of C-peptide in their blood, than those on placebo. This is a significant finding pointing to the potential application of these therapies to T1D.
According to a new publication made possible by JDRF support, “modest concentrations of C-peptide in type 1 diabetes are associated with better clinical outcomes including reductions in hypoglycemia, neuropathy, and retinopathy.” More C-peptide, better outcomes.
Here’s a rundown of several DMTs that, through JDRF-funded studies, have the potential to join Tzield as an approved DMT for T1D.
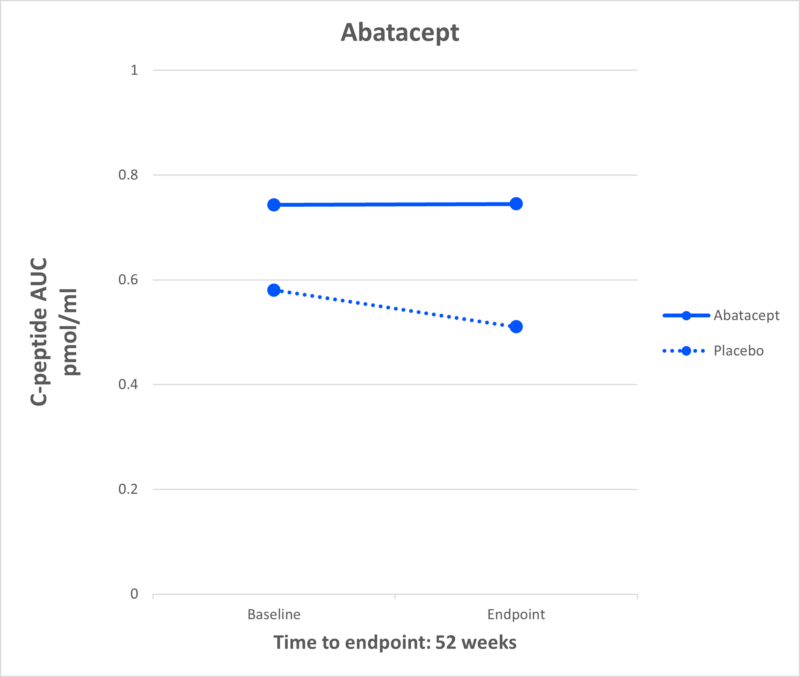
Abatacept
What It Is: Abatacept is a drug that reduces inflammation by inhibiting the activity of the T cells. It is currently approved for use in autoimmune disorders, like rheumatoid arthritis.
Effect in T1D: In 2011, a JDRF-funded study showed that individuals receiving abatacept had higher levels of C-peptide compared to placebo.
Current Research: JDRF is funding a follow-up study in Australia that uses a nasal insulin as a tolerizing agent to see if using tolerizing agents and immunotherapies at the same time can lead to longer-lasting results.
JDRF Role: JDRF has supported the development of abatacept for over 15 years and is currently funding a follow-up study.
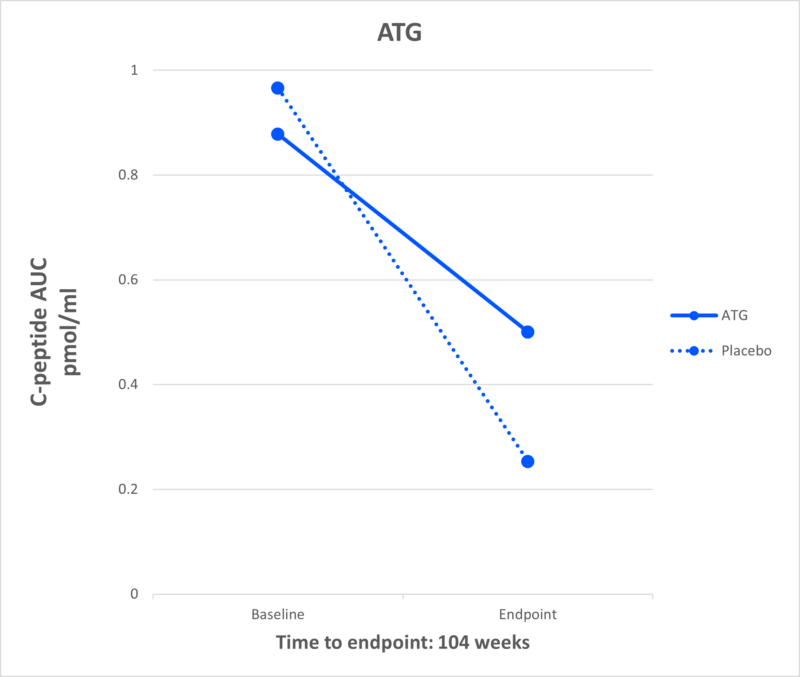
ATG
What It Is: Anti-thymocyte globulin (ATG) is an FDA-approved drug used to increase white blood cell counts in individuals receiving chemotherapy or transplantation.
Effect in T1D: The Diabetes TrialNet ATG-GCSF New Onset Study found that, after two years, low-dose ATG preserved beta cell function and improved insulin production in individuals newly diagnosed with T1D. People on ATG-alone also had lower A1c levels.
Current Research: There are several JDRF-supported studies continuing this research. This includes:
- MELD-ATG – a phase II study conducted by INNODIA, a JDRF-supported public/private partnership, to investigate the effect of low-dose ATG on C-peptide at 12 months compared to placebo.
- STOP-T1D – a phase II study conducted by TrialNet, an SDP-funded and JDRF-supported network at the forefront of T1D research, investigating if a low dose of ATG can delay or prevent T1D in people ages 12 and up to 35 who have a 50% risk of clinical diagnosis (Stage 3) within 2 years.
JDRF Role: JDRF has supported the development of ATG for over 10 years and is currently funding follow-up studies.
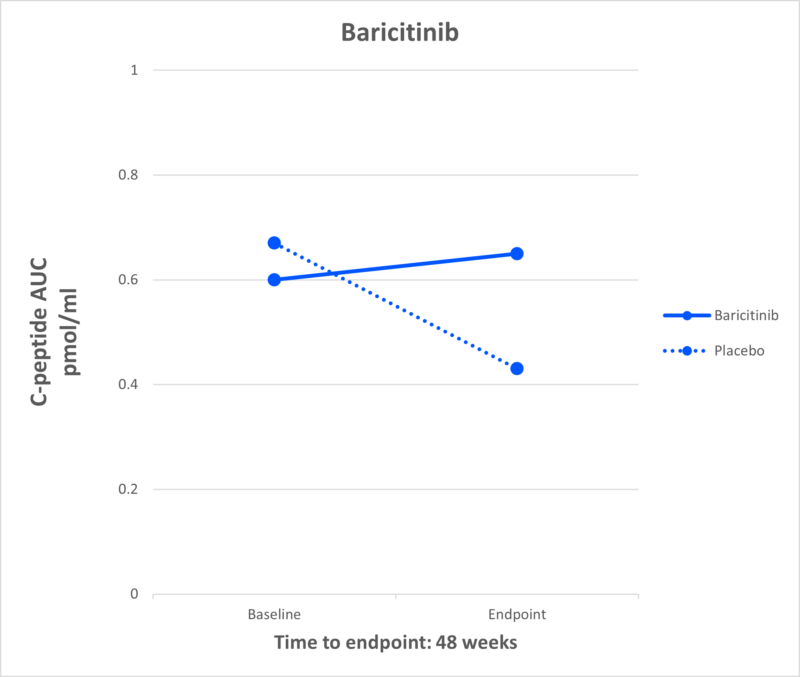
Baricitinib
What It Is: Baricitinib is a JAK inhibitor currently approved for use in autoimmune disease, including rheumatoid arthritis and alopecia.
Effect in T1D: In a JDRF-funded clinical trial published in the renowned New England Journal of Medicine, investigators demonstrated that baricitinib—a JAK inhibitor, which is critical to signaling pathways within both immune cells and beta cells in T1D—preserved beta cell function in the disease.
Current Research: There are several clinical trials to see if JAK inhibitors, like baricitinib, can be effective if used in conjunction with other therapies, such as Tzield or verapamil, in presymptomatic disease, or in longer duration.
JDRF Role: JDRF, along with JDRF Australia, funded the seminal BANDIT study which showed that baricitinib can preserve insulin production in adolescents and teens recently diagnosed with T1D.
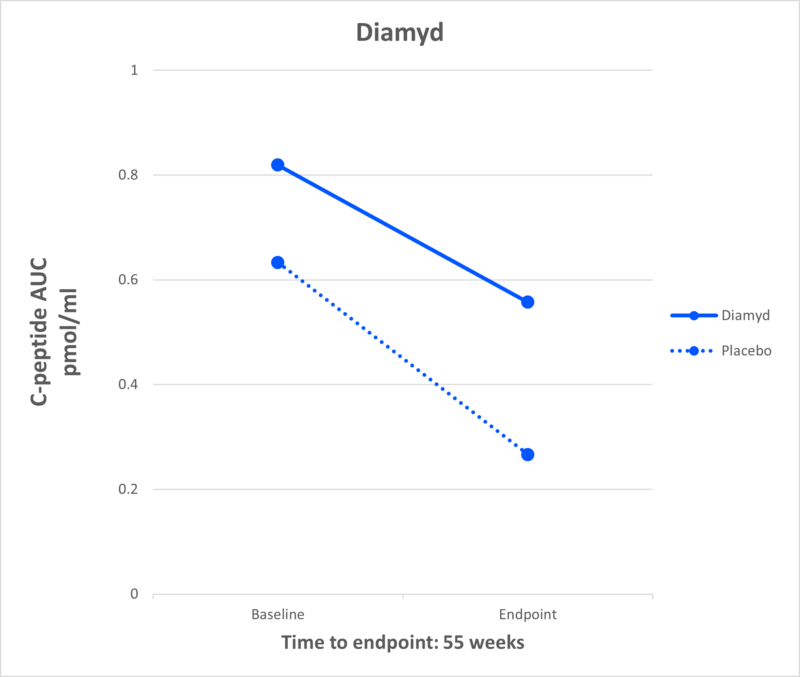
Diamyd
What It Is: Diamyd is an antigen-specific immunotherapy, directed toward the autoantigen GAD, to stop the autoimmune destruction of insulin-producing cells. Based upon strong clinical evidence, this therapy is the first to be targeted to a specific T1D sub-population: individuals with the HLA DR3-DQ2 gene, a genetic risk factor for T1D, and presence of antibodies to the GAD antigen. Collectively, this accounts for almost 40% of people with newly diagnosed T1D.
Effect in T1D: In a Phase I/II study, Diamyd demonstrated statistically significant and clinically meaningful beneficial effects on preservation of insulin production and blood glucose control.
Current Research: Diamyd is conducting the DIAGNODE-3 Diabetes Study which will test the safety and efficacy of the therapy in approximately 330 adolescents and young adults aged 12-29 recently diagnosed with T1D.
JDRF Role: JDRF is funding the DIAGNODE-3 Diabetes study.
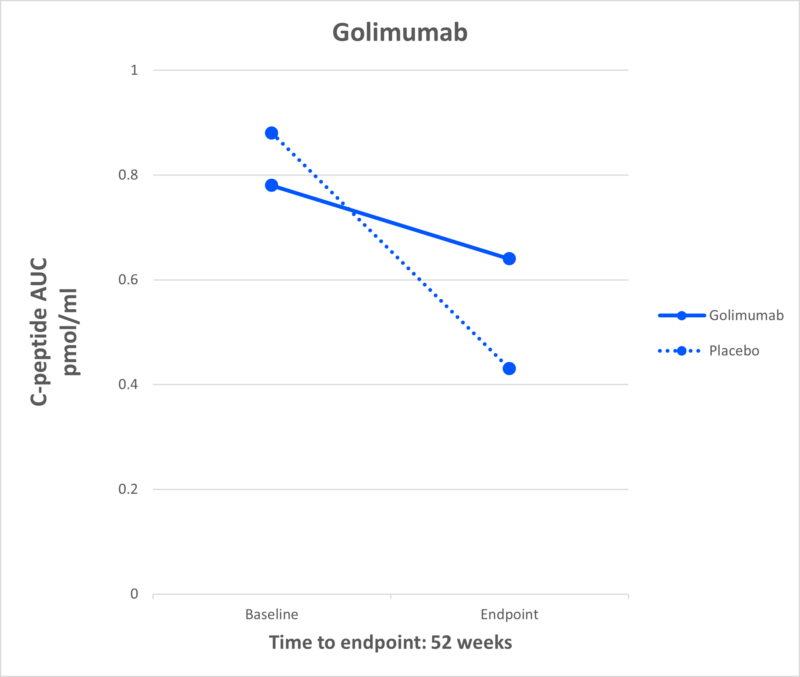
Golimumab
What It Is: A monoclonal antibody that inhibits TNF alpha, a chemical messenger that leads to inflammation and increases the immune response to beta cells.
Effect in T1D: Results published in the New England Journal of Medicine in 2020 demonstrated children and adolescents newly diagnosed with T1D who received golimumab produced more insulin compared to placebo.
Current Research: JDRF is pursuing this class of drugs to see potential future uses in DMT.
JDRF Role: JDRF has partnered with other funders to validate the results of this study in combination with another therapy that also preserves C-peptide.
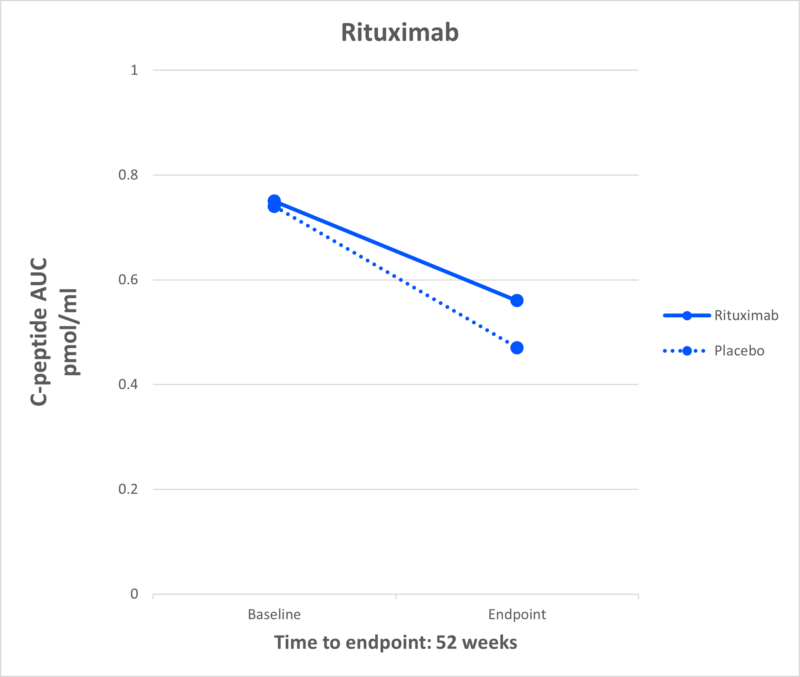
Rituximab
What It Is: Rituximab is a monoclonal antibody medication used to treat certain autoimmune diseases, like rheumatoid arthritis, and types of cancers. It is the only one of the DMTs discussed in this piece that target B cells, the cells that activate the cells that destroy the beta cells.
Effect in T1D: A study by TrialNet showed that rituximab preserved beta cell health in individuals recently diagnosed with T1D.
Current Research: TrialNet is conducting the T1D RELAY study to see if two immune therapies that have shown benefit in T1D—rituximab-pvvr, followed by abatacept—can together preserve insulin production in people recently diagnosed with T1D.
JDRF Role: JDRF’s work is closely aligned with TrialNet in efforts to develop DMTs for T1D. JDRF is currently supporting research into B cells, which is what rituximab targets.
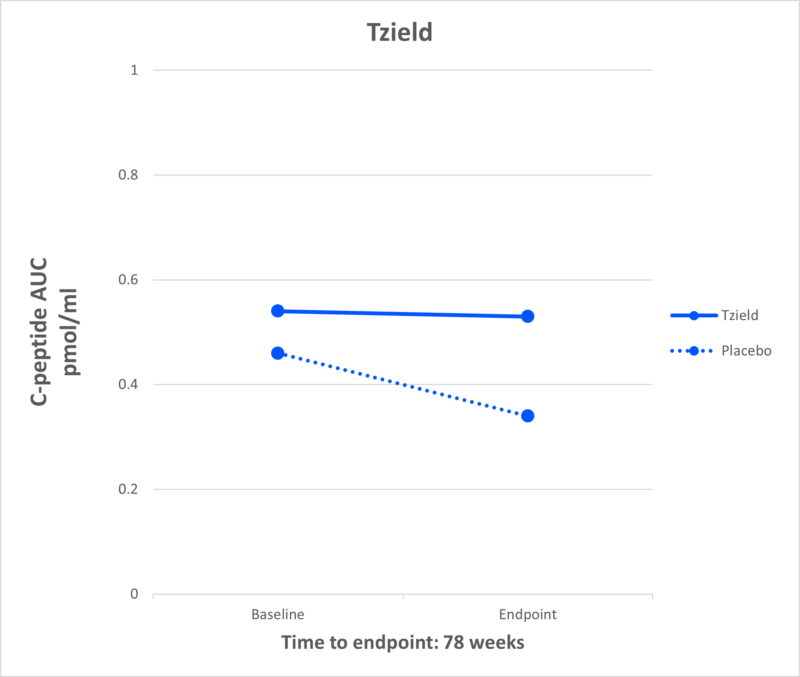
Tzield
What It Is: Tzield (teplizumab-mzwv) is an anti-CD3 monoclonal antibody, FDA approved to delay onset of T1D in at-risk (stage 2) individuals.
Effect in T1D: Tzield delays the progression of T1D by a median of 2.7 years in individuals with multiple autoantibodies and abnormal blood sugar levels. Tzield is the first FDA-approved DMT for T1D.
The results of the PROTECT study, which were recently published in the New England Journal of Medicine, showed that newly-diagnosed individuals can also benefit from this therapy.
Current Research: There are multiple studies, including the Ver-a-T1D trial, to see if Tzield can be used in combination with other therapies to delay T1D progression.
JDRF Role: JDRF has supported the development of teplizumab for more than 30 years.
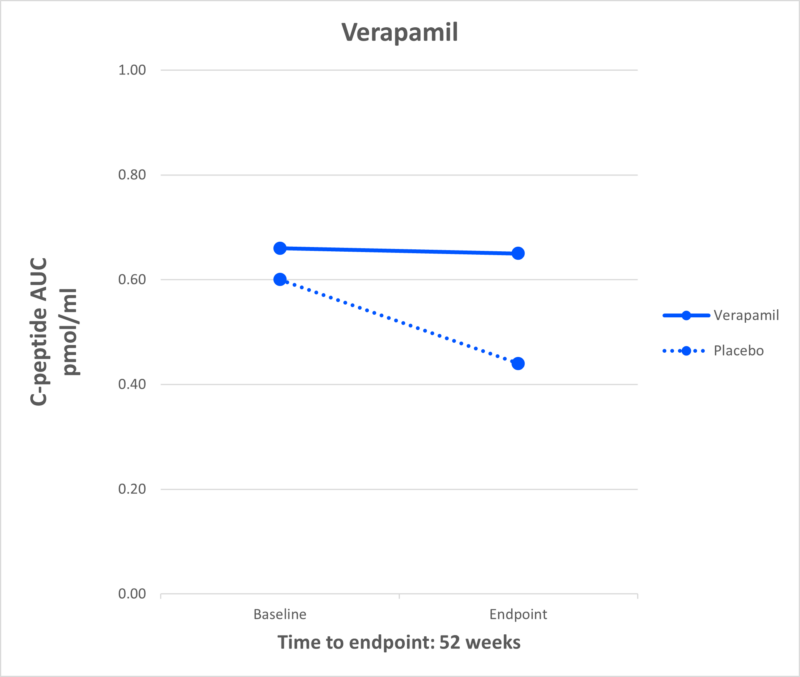
Verapamil
What It Is: Verapamil is a generic calcium channel blocker used primarily to treat high blood pressure.
Effect in T1D: Multiple JDRF-funded studies, including the CLVer study, have shown that verapamil can preserve beta cells in newly diagnosed individuals.
Current Research: JDRF is currently funding a follow-up study for three years to see if C-peptide benefits persist. Additionally, JDRF is funding several clinical trials to validate the results of this study and see if verapamil is effective when used in conjunction with other disease-modifying therapies, such as Tzield.
JDRF Role: JDRF has supported research into verapamil for more than 20 years.
What Comes Next for Disease-Modifying Therapies?
The journey from discovery research to regulatory approval and global access to a drug is long and expensive. Very few therapies make it all the way through because of the vast evidence and resources required.
“JDRF wants new therapies to prove safe and effective and get through the regulatory process as efficiently as possible,” said Campbell Hutton, JDRF vice president, regulatory and health policy. “We have a team at JDRF that works with regulators and industry to ensure this happens. We help establish regulatory pathways, update regulators on the state of T1D science so they are ready to review these new therapies, make sure there is a common understanding of the risks and benefits of new therapies, and more.”
The clinical data reviewed above is critically important for future regulatory approvals. Thanks to dedicated researchers and the contributions of the JDRF community, we have significant amounts of data that show the promise of these therapies for people with T1D or at-risk for developing it. However, to achieve regulatory approval, the data, in some cases, will need to be replicated with more people for a longer time.
Even after having the clinical trial data needed, a sponsor, or the company who makes the drug, needs to submit the data and other information, like on the manufacturing of the drug, to FDA (the regulatory agency in the U.S.) for it to be reviewed. If a drug is owned by a single pharmaceutical company, they are responsible for submitting the application as the sponsor. If a drug’s patent has expired and is made by multiple companies, it can be challenging to find a company willing to engage in the long and expensive regulatory process.
JDRF plays a key role here, encouraging industry to support clinical research and take the necessary steps to get these therapies to market.
As you can see, this is a complicated process, which is why JDRF has filled the pipeline with therapies that all have a decent shot of making it to market.
JDRF Enabling Progress in Disease-Modifying Therapies
JDRF is the only organization that supports therapy development at every stage—from discovery research all the way through to people with diabetes receiving these therapies—with healthcare coverage paying for them. This is a lengthy and expensive process; success requires many stakeholders to come together toward a common goal. JDRF plays a crucial role here to ensure this collaboration happens and everyone, from researchers to regulators to payers—are all pulling in the same direction.
JDRF is committed to having multiple shots on goal to cure T1D. The DMT pipeline, as evidenced by the eight DMT candidates above, is proof of that commitment.
Hopefully in the future one of these, or other therapies, will join Tzield and give the T1D community another option for delaying, halting progression, or eventually reversing this disease.
Key Terms to Know
We know T1D research has its own language. Here’s a quick refresher on frequently-used terms.
Antibody – blood protein created in response to an antigen
Antigen – something that causes an immune response
Beta cells – cells in the pancreas that produce insulin. These are the cells destroyed by the immune system in people with T1D.
C-peptide – a biomarker that indicates the presence of insulin production. When the body produces insulin, it also creates C-peptide.
HbA1c – a blood test used to measure average blood glucose levels over the past three months.
Immunotherapy – drug aimed at altering the body’s immune system. Specifically, immunotherapies for T1D are intended to disrupt the autoimmune attack behind T1D. Also called “immune therapies.”
Placebo – A treatment, such as a dummy pill, that does not contain any of the drug being tested.